Question
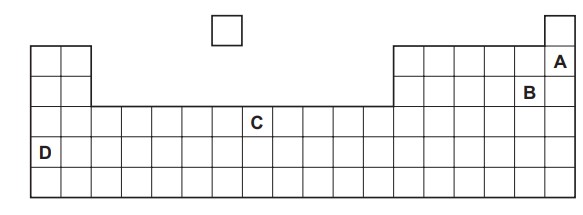
Part of the Periodic Table is shown. Which element is a soft solid that reacts violently with cold water?
▶️Answer/Explanation
Ans:
D
D is a alkali metal. It is a soft solid. It reacts violently with cold water.
Question
A Group I metal (lithium, sodium or potassium) is reacted with a Group VII element (chlorine, bromine or iodine).
Which compound is formed when the Group I metal of highest density reacts with the Group VII element of lowest density?
A lithium chloride
B potassium chloride
C potassium iodide
D lithium iodide
▶️Answer/Explanation
Ans:B
Group I metal of highest density is potassium and Group VII element of lowest density is chlorine. Together, they react to form KCl.
Question
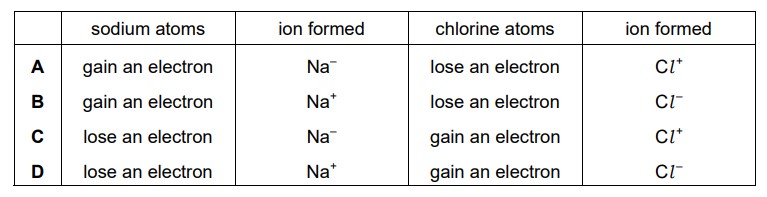
Sodium is in Group I of the Periodic Table and chlorine is in Group VII. Which row describes what happens when sodium bonds ionically with chlorine?
▶️Answer/Explanation
Ans:
D
When sodium (Na) bonds ionically with chlorine (Cl), they form an ionic compound known as sodium chloride (NaCl), which is commonly known as table salt. Ionic bonding occurs when there is a transfer of electrons from one atom to another.
In the case of sodium chloride, sodium (Na) has one valence electron in its outermost shell, while chlorine (Cl) has seven valence electrons. Sodium readily loses its valence electron, becoming a positively charged ion (Na+), while chlorine gains the electron, becoming a negatively charged ion (Cl–).
Question
Sodium is in Group I of the Periodic Table.
Chlorine is in Group VII of the Periodic Table.
Sodium and chlorine combine to form a compound.
Which statement about the combination of sodium and chlorine atoms is correct?
A Both sodium and chlorine lose electrons.
B Both sodium and chlorine gain electrons.
C Sodium loses electrons and chlorine gains electrons.
D Sodium gains electrons and chlorine loses electrons.
▶️Answer/Explanation
Ans:C
When sodium (Na) bonds ionically with chlorine (Cl), they form an ionic compound known as sodium chloride (NaCl), which is commonly known as table salt. Ionic bonding occurs when there is a transfer of electrons from one atom to another.
In the case of sodium chloride, sodium (Na) has one valence electron in its outermost shell, while chlorine (Cl) has seven valence electrons. Sodium readily loses its valence electron, becoming a positively charged ion (Na+), while chlorine gains the electron, becoming a negatively charged ion (Cl–).
Question
Rubidium is in Group I of the Periodic Table and bromine is in Group VII.
Rubidium reacts with bromine to form an ionic compound.
Which row shows the electron change taking place for rubidium and the correct formula of the rubidium ion?

▶️Answer/Explanation
Ans:C
Rb loses one electron to form Rb+ ion. Br gains one electron to form Br– anion.