Question
Some properties of the elements in Group VII of the Periodic Table are shown.
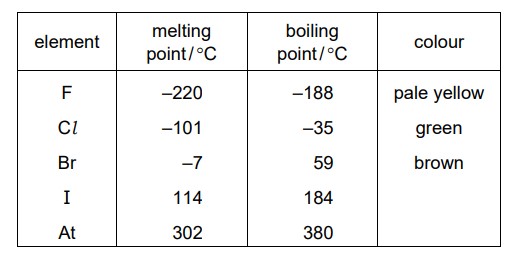
Which statement is correct?
A. Bromine is a brown solid at room temperature.
B. Fluorine is a pale yellow gas at room temperature.
C. Iodine is a brown liquid at room temperature.
D. Astatine is a black liquid at room temperature.
▶️Answer/Explanation
Ans:
B
At room temperature and pressure (around 25 degrees Celsius and 1 atmosphere), fluorine is a pale yellow gas with a pungent odor.
Question
Two tests are carried out on substance $Z$.
test 1 A flame test produces a red flame.
test $2 \mathrm{Z}$ is dissolved in water and dilute nitric acid is added, followed by aqueous silver nitrate. A yellow precipitate is produced.
What is substance $\mathrm{Z}$ ?
A lithium bromide
B lithium iodide
C sodium bromide
D sodium iodide
▶️Answer/Explanation
Ans:B
When lithium compounds, such as lithium iodide, are heated in a flame, the thermal energy excites the electrons in the lithium atoms. As the electrons return to their ground state, they release energy in the form of light. In the case of lithium ions, the emitted light falls in the range of crimson red or magenta, which can be observed during the flame test.
When lithium iodide is dissolved in water and dilute nitric acid is added, followed by aqueous silver nitrate, a yellow precipitate is produced. In this reaction, silver ions from silver nitrate react with iodide ions from lithium iodide to form silver iodide as a solid precipitate. Silver iodide is a yellow compound, which explains the appearance of a yellow precipitate when silver nitrate is added.
Question
Which statement about elements in Group I and Group VII of the Periodic Table is correct?
A. Bromine reacts with potassium chloride to produce chlorine.
B. Iodine is a monoatomic non-metal.
C. Lithium has a higher melting point than potassium.
D. Sodium is more reactive with water than potassium.
▶️Answer/Explanation
Ans:
C
Lithium has high melting and boiling point because of the high ionization enthalpy and high crystal lattice binding energy.
Question
Astatine is below iodine in Group VII in the Periodic Table.
Which row describes the properties of astatine?

▶️Answer/Explanation
Ans:
D
Astatine (At) is a radioactive element that is typically considered a solid at room temperature.
At room temperature, astatine does not readily displace chlorine (Cl), bromine (Br), or iodine (I) from their compounds.
Astatine is a highly reactive halogen, but it is the least reactive among the halogens due to its large atomic size and high electronegativity. Its reactivity decreases as you move down the halogen group in the periodic table.
Question
Which row describes the trends in the properties of the Group VII elements as the group is descended?
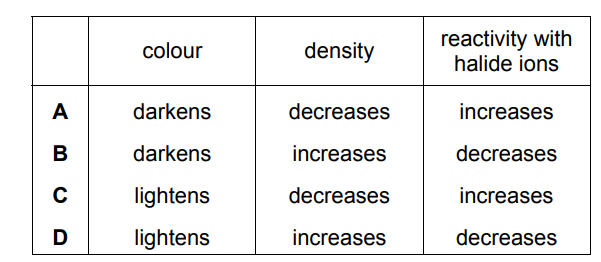
▶️Answer/Explanation
Ans:B
The halogens exhibit a trend in color as you descend the group. Fluorine is a pale yellow gas, chlorine is a greenish-yellow gas, bromine is a reddish-brown liquid, iodine is a purple-black solid, and astatine is expected to be a dark, lustrous solid.
The density of the halogens generally increases as you descend the group. Fluorine and chlorine, as gases, have low densities. Bromine is a liquid and has a higher density than the gases. Iodine, as a solid, has an even higher density. Astatine is expected to have a higher density than iodine.
The reactivity of the halogens also follows a trend as you descend the group. Fluorine is the most reactive of the halogens, readily undergoing reactions with many substances. Chlorine is also highly reactive but less so than fluorine. Bromine is less reactive than both fluorine and chlorine but still reactive under appropriate conditions. Iodine is less reactive than the previous three halogens. Astatine is expected to be the least reactive halogen due to its large atomic size and low reactivity.