Question
A metal reacts vigorously with cold water. Which statement about the metal is correct?
A. It is above hydrogen in the reactivity series.
B. It is below magnesium in the reactivity series.
C. Its oxide can be reduced with carbon.
D. It does not react with dilute acids.
▶️Answer/Explanation
Ans:
A
If a metal reacts vigorously with cold water, it indicates that the metal has a higher reactivity than hydrogen. In the reactivity series, metals are listed based on their ability to displace hydrogen from water or acids. Metals that are above hydrogen in the reactivity series will react with water, displacing hydrogen gas and forming metal hydroxides. Metals that are below hydrogen in the reactivity series will not react with water but may react with acids.
Question
A reactivity series is shown.
sodium
calcium
magnesium
carbon
zinc
iron
hydrogen
copper
Which statement is correct?
A All the metals above carbon are extracted by electrolysis.
B Iron can only be extracted by electrolysis.
C Calcium can be extracted by heating calcium oxide with carbon.
D Copper can only be extracted by passing hydrogen over heated copper(II) oxide.
▶️Answer/Explanation
Ans:A
Metals above carbon (sodium, calcium, magnesium) are very reactive and are usually extracted using electrolysis, which involves passing an electric current through a molten compound of the metal or its aqueous solution to break it down and obtain the pure metal.
Question
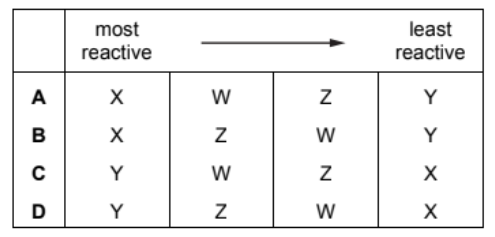
The properties of four metals, $\mathrm{W}, \mathrm{X}, \mathrm{Y}$ and $\mathrm{Z}$, are shown.
W It does not react with cold water but reacts with steam.
$X$ It does not react with water or dilute acid but the oxide of $X$ is reduced by carbon.
$Y$ The oxide of $Y$ is not reduced by carbon but $Y$ reacts vigorously with cold water.
Z It does not react with water or steam but reacts with dilute acid.
What is the order of reactivity of the elements starting with the most reactive?
▶️Answer/Explanation
Ans:C
The correct order of reactivity from the most reactive to the least reactive, based on the information provided, is:
- Y: It reacts vigorously with cold water.
- W: It does not react with cold water but reacts with steam.
- Z: It does not react with water or steam but reacts with dilute acid.
- X: It does not react with water or dilute acid, but the oxide of X is reduced by carbon.
So, the correct order of reactivity is indeed Y > W > Z > X.
Question
The metal beryllium does not react with cold water.
It reacts with hydrochloric acid but cannot be extracted from its ore by using carbon.
Where is beryllium placed in the reactivity series?
magnesium
A
zinc
B
iron
C
copper
D
▶️Answer/Explanation
Ans: A
Since beryllium reacts with hydrochloric acid but cannot be extracted from its ore using carbon, it must be placed above zinc in the reactivity series. This means that beryllium is more reactive than zinc.
Question
Manganese, nickel and silver are all metals.
Samples of powdered manganese, nickel and silver were placed in separate test-tubes
containing dilute hydrochloric acid.
The results are shown.
What is the order of reactivity of the metals, most reactive to least reactive?
A manganese → nickel → silver
B manganese → silver → nickel
C silver → manganese → nickel
D silver → nickel → manganese
▶️Answer/Explanation
Ans: A
Based on the observations of the reaction with dilute hydrochloric acid, the order of reactivity of the metals from most reactive to least reactive is as follows:
- Manganese (Mn): It reacts with dilute hydrochloric acid.
- Nickel (Ni): It also reacts with dilute hydrochloric acid, but it is less reactive than manganese.
- Silver (Ag): It does not react with dilute hydrochloric acid and is the least reactive among the three metals.
So, the order of reactivity is Manganese (most reactive) > Nickel > Silver (least reactive).