Question
The diagrams show experiments to investigate rusting of iron nails.

In which test-tubes do the nails rust?
A 1 only
B 1 and 2 only
C 1 and 3 only
D 1,2 and 3
▶️Answer/Explanation
Ans:B
Rust is formed when iron reacts with oxygen and water to create iron oxide. Boiling water eliminates dissolved oxygen, which is a crucial component in the rusting process. When the water is boiled, the oxygen molecules present in the water escape as gas, leaving behind oxygen-depleted water. Also, its top is also closed, so atmospheric air cannot enter.
Question
Which pollutants are responsible for the erosion of buildings and statues?
1 carbon monoxide
2 oxides of nitrogen
3 sulfur dioxide
A 1, 2 and 3 B 1 and 2 only C 2 and 3 only D 3 only
▶️Answer/Explanation
Ans: C
Acid rain is formed when sulfur dioxide and nitrogen oxides react with water, oxygen, and other chemicals in the atmosphere. It creates acidic precipitation, which can cause significant damage to buildings and statues made of limestone, marble, and other calcareous stones. Acid rain reacts with the calcium carbonate present in these stones, dissolving and eroding the surfaces.
Question
Which row identifies a use of mild steel and a use of stainless steel?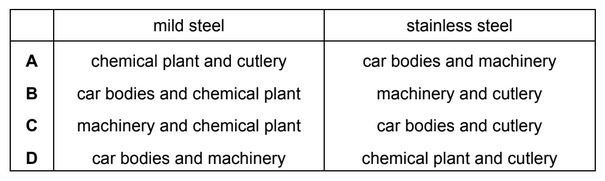
▶️Answer/Explanation
Ans: D
Mild steel is a popular material used in car bodies and machinery due to its favorable mechanical properties, cost-effectiveness, and ease of fabrication. It is a type of carbon steel with low carbon content (generally less than 0.25%), making it relatively ductile and malleable.
Stainless steel’s combination of corrosion resistance, strength, hygiene, and aesthetic appeal makes it a versatile and widely used material in both chemical plants and cutlery applications.
Question
Which methods prevent iron from rusting?
▶️Answer/Explanation
Ans: C
Saltwater can accelerate the rusting process of nails (and other metals) compared to regular freshwater. The reason behind this lies in the corrosive nature of saltwater due to its high concentration of dissolved salts, particularly sodium chloride (NaCl).
Question
The diagram shows an experiment to investigate how paint affects the rusting of iron.

What happens to the water level in tubes P and Q?

▶️Answer/Explanation
Ans:
D
Paint acts as a protective barrier, creating a physical barrier between the iron surface and the external environment, which includes oxygen and moisture – the primary factors that contribute to rust formation. So, water level in tube Q doesn’t change.