Question
This question is about air.
(a) The pie chart shows the proportions of the main gases in clean, dry air.

(i) Name the gases G and H.[2]
gas G
gas H
(ii) The graph shows how the volume of a sample of gas G changes as temperature increases. The pressure is kept constant.

Describe how the volume of gas G changes as temperature increases.[1]
(iii) There is a small percentage of noble gases in the air.
The noble gases are unreactive.
Explain why the noble gases are unreactive in terms of their electronic structure.[1]
(iv) Describe the arrangement and separation of the particles in a gas.[2]
arrangement
separation
(b) Two of the pollutants in air are oxides of nitrogen and lead compounds.
(i) Give one effect of each of these pollutants on health.[2]
oxides of nitrogen
lead compounds
(ii) Name two other pollutants present in air.
State the source of each of these pollutants.[4]
pollutant 1
source of pollutant 1
pollutant 2
source of pollutant 2 [Total: 12]
Answer/Explanation
Ans:
(a)(i) G is oxygen (1)
H is nitrogen (1)
(a)(ii) volume increases (as temperature increases)
(a)(iii) they have a full outer shell (of electrons) / they have a complete outer shell (of electrons)
(a)(iv) arrangement: irregular / random (1)
separation: far apart (1)
(b)(i) oxides of nitrogen:
breathing difficulties / irritates lungs / irritates eyes / irritates throat / irritates skin / lung problems (1)
lead compounds:
poisonous / toxic / harms nervous system / harms brain (1)
(b)(ii) 1 mark for each correct pollutant and one 1 mark for each correct source e.g.
sulfur dioxide (1)
burning fossil fuels / volcanoes (1)
carbon monoxide (1)
incomplete combustion of carbon containing substance / incomplete combustion of named carbon compound (1)
Question
The table shows the mass of air pollutants, in nanograms, in $1000 \mathrm{~cm}^3$ samples of air taken over a four month period.
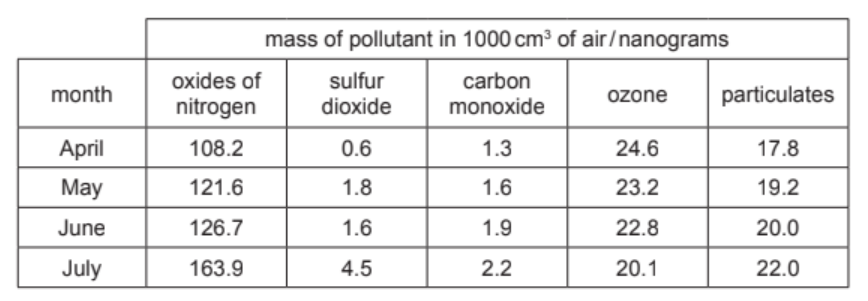
(a) Answer these questions using only the information in the table.
(i) Name the pollutant that shows a decrease in concentration between April and July.[1]
(ii) Name the pollutant present in the lowest concentration in May.[1]
(iii) Calculate the mass of sulfur dioxide in $250 \mathrm{~cm}^3$ of the sample of air taken in April.
nanograms [1]
(b) Oxides of nitrogen are produced when oxygen combines with nitrogen during thunderstorms.
(i) State one other source of oxides of nitrogen in the air.[1]
(ii) Give one adverse effect of oxides of nitrogen on health.[1]
(iii) Complete the chemical equation for the reaction of nitrogen with oxygen to form nitrogen dioxide.
$
+2 \mathrm{O}_2 \rightarrow \ldots . . \mathrm{NO}_2
$
(c) Particulates are tiny solid particles in the air.
The movement of these particles is shown by the arrows in the diagram.
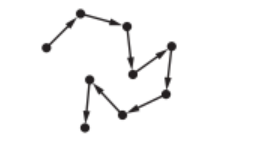 State the name given to this random motion of particles.
State the name given to this random motion of particles.
……………………………………………………………………………………………………………………………. [1] [Total: 8]
▶️Answer/Explanation
Ans:
(a)(i) ozone
a)(ii) carbon monoxide / CO
(a)(iii) 0.15 (ng)
(b)(i) car exhausts / car engines / high temperature furnaces
(b)(ii) irritation of lungs / irritates nose / irritates throat / irritates eyes / asthma
(b)(iii) $\begin{aligned} & \mathrm{N}_2 \text { (on left) } \\ & 2\left(\mathrm{NO}_2\right) \text { (on right) }\end{aligned}$
(c) Brownian (motion)
Question
This question is about sulfur, sulfur compounds and the water from a sulfur spring. A sulfur spring is a natural source of water containing sulfur.
(a) The table shows the mass of ions present in a 1000cm3 sample of water from a sulfur spring.
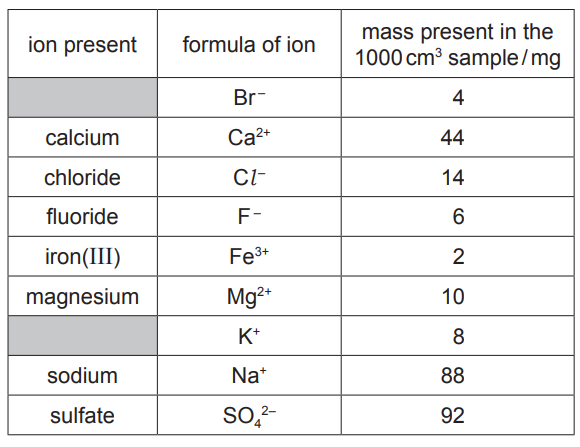
Answer these questions using only information from the table.
(i) Which negative ion is present in the lowest concentration?[1]
(ii) Give the name of the compound formed from only K+ and Br– ions.[1]
(iii) Calculate the mass of calcium ions present in a 250cm3 sample of this water.
mass of calcium ions = mg [1]
(iv) Complete the equation to show the formation of a fluoride ion from a fluorine atom.[1]
F + ____ → F–
(b) Describe a test for sulfate ions.[2]
test
observations
(c) Solid sulfur is found around the edge of sulfur springs.
(i) When heated, sulfur undergoes sublimation.
What is meant by the term sublimation?[1]
(ii) Sulfur reacts with hot concentrated sulfuric acid.
Complete the chemical equation for this reaction.
S + ___ H2SO4 2H2O + ___ SO2 [2]
(iii) The table shows the solubility of sulfur and zinc in an organic solvent and in water. The organic solvent boils at 46°C.
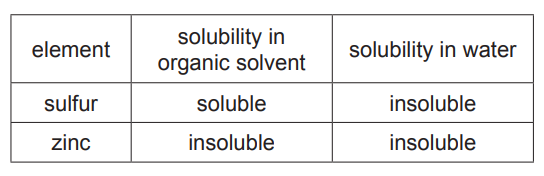
Use the information in the table to suggest how to obtain pure, dry samples of both sulfur and zinc from a mixture of sulfur powder and zinc powder.[4]
(d) The structure of a sulfur compound is shown.
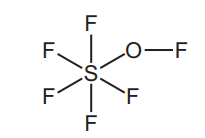
Deduce the molecular formula of this compound showing the number of sulfur, fluorine and oxygen atoms.[1][Total: 14]
Answer/Explanation
Ans:
(a)(i) Br– / bromide
(a)(ii) potassium bromide
(a)(iii) 11 (mg)
(a)(iv) e–
(b) (acidified and add aqueous) barium chloride / barium nitrate forms white precipitate (2)
if 2 marks not scored: 1 mark for barium chloride / barium nitrate
(c)(i) idea of solid turning (directly) to gas 1
(c)(ii) 2 (H2SO4) (1)
3 (SO2) (1)
(c)(iii)
- add (organic) solvent to the mixture (and stir until all sulfur dissolves) (1)
- filter off the zinc / filter off the residue (1)
- let solvent evaporate from zinc / residue (1)
- evaporate the sulfur solution / evaporate the solvent from the solution / evaporate solvent from filtrate (1)
(d) SF6O