Question
This question is about Group IV elements and their compounds.
(a) The changes of state of lead are shown.

Name the changes of state represented by A and B.[2]
A
B
(b) Use the kinetic particle model to describe the differences between liquid lead and lead gas in terms of:[4]
-
- the separation of the particles
- the motion of the particles.
(c) Lead is extracted from lead(II) oxide by heating with carbon.
PbO + C → Pb + CO
Describe how this equation shows that lead(II) oxide is reduced.[1]
(d) Lead is a pollutant of the air.
(i) State one source of lead in the air.[1]
(ii) State one adverse effect of lead on health.[1]
(e) Diamond is a form of carbon.
The structure of diamond is shown.

(i) Choose the word which best describes the structure of diamond.
Draw a circle around your chosen answer.[1]
giant ionic metallic simple
(ii) Name the type of bonding in diamond.[1]
(iii) Give one use of diamond.[1]
(iv) Deduce the electronic structure of carbon.
Use the Periodic Table to help you.[1] [Total: 13]
Answer/Explanation
Ans:
a) A: freezing (1)
B: boiling (1)
b) separation:
liquid: close together / some touching (1)
gas: far apart (1)
motion:
liquid: sliding over each other / restricted movement (1)
gas: moving freely (1)
(c) lead oxide has lost oxygen
(d)(i) (leaded) petrol / paints
(d)(ii) poisonous / toxic / harms nerves / harms brain
(e)(i) giant
(e)(ii) covalent
(e)(iii) cutting tools / for cutting / drill tips / jewelry
(e)(iv) 2,4
Question
The diagram shows a blast furnace used in the extraction of iron.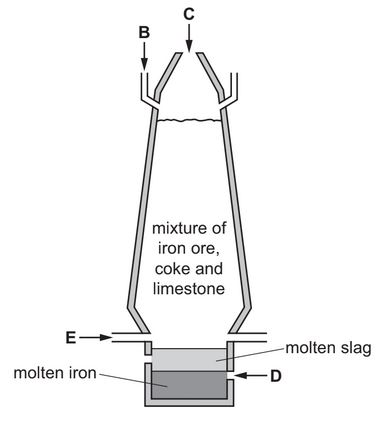
(a) Air is blown into the furnace.
State which letter on the diagram, B, C, D or E, shows where air is blown into the furnace.
(b) (i) Complete the chemical equation for the reduction of iron(III) oxide in the blast furnace.
\(Fe_2O_3 + 3C → ……Fe + ……CO\)
(ii) Explain how this equation shows that iron(III) oxide is reduced.
(c) Calcium carbonate (limestone) is added to the blast furnace.
The calcium carbonate undergoes thermal decomposition.
(i) Complete the word equation for this reaction.
(ii) One of the products of this reaction reacts with impurities in the iron to form slag.
Use the information in the diagram to suggest how you know that molten slag is less dense than molten iron.
(d) (i) Use words from the list to complete these sentences about how steel is made from iron.
acidic basic chlorides methane neutral
nitrogen oxides oxygen sulfates
A gas is blown through the molten iron. The name of this gas is …………………… .
Acidic gases are formed. These acidic gases react with …………………… ……………………
(ii) State one use of mild steel.
(iii) Metals such as chromium are added to iron to make stainless steel.
The symbol for an isotope of chromium is shown.
\(_{24}^{53}Cr\)
Deduce the number of electrons, neutrons and protons in one atom of this isotope of
chromium.
number of electrons ………………………………………………………………………………………………
number of neutrons ………………………………………………………………………………………………
number of protons ………………………………………………………………………………………………..
(e) Chromium conducts electricity and is shiny.
Give two other physical properties of chromium that are characteristic of all metals.
1 ……………………………………………………………………………………………………………………………….
2 ……………………………………………………………………………………………………………………………….
Answer/Explanation
Answer:
(a) E
(b) (i) 2 (Fe) (1)
3 (CO) (1)
(ii) iron oxide loses oxygen / it loses oxygen
(c) (i) calcium oxide (1)
carbon dioxide (1)
(ii) slag floats above the iron
(d) (i) oxygen (1)
basic (1) oxides (1)
(ii) car bodies / machinery
(iii) electrons: 24 (1)
neutrons: 29 (1)
protons: 24 (1)
(e) 1 mark each for any two of:
• conducts heat
• malleable
• ductile
Question
Water is essential for many industrial processes.
(a) State one use of water in industry.
(b) What is the pH of pure water?
Draw a circle around the correct answer.
pH 0 pH 6 pH 7 pH 14
(c) Filtration and chlorination are two of the steps used in water treatment.
Describe the purpose of each of these steps.
filtration ……………………………………………………………………………………………………………………..
chlorination ………………………………………………………………………………………………………………..
(d) The changes of state of water are shown.
Give the names of the changes of state represented by A and B.
A ………………………………………………………………………………………………………………………………
B ………………………………………………………………………………………………………………………………
(e) The table compares the reactions of four metals with both steam and dilute hydrochloric acid.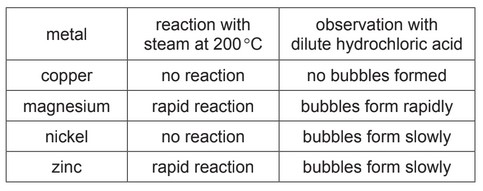
Put the four metals in order of their reactivity.
Put the least reactive metal first.
Answer/Explanation
Answer:
(a) coolant / cooling / solvent / for dissolving
(b) pH 7
(c) filtration: to remove solids / to remove insoluble materials / to separate solids from liquids (1)
chlorination: to kill bacteria / to disinfect (the water) / to kill (harmful) microorganisms (1)
(d) A: melting / fusion (1)
B: condensing / condensation (1)
(e) copper <nickel <zinc <magnesium (2)
if two marks not scored one mark for one consecutive pair reversed / all reversed