Question
The table shows some properties of four Group I elements.
(a) (i) Complete the table by predicting:
● the boiling point of rubidium
● the atomic radius of potassium.
(ii) Describe the trend in the melting point of the Group I elements down the group.
(iii) Deduce the physical state of potassium at 60°C.
Explain your answer.
(b) Caesium is a radioactive element with a proton number of 55.
(i) Define proton number.
(ii) State one industrial use of radioactive isotopes.
(c) Sodium hydride, NaH, reacts with iron(III) oxide.
(i) Balance the equation for this reaction.
\(Fe_2O_3 + 3NaH → …..Fe + …..NaOH\)
(ii) Explain how this equation shows that iron(III) oxide is reduced.
Answer/Explanation
Answer:
(a) (i) boiling point of Rb: any values between 675 and 755 (°C) (inclusive of these values) (1)
atomic radius of K: any values between 0.195 and 0.245 (nm) (inclusive of these values) (1)
(ii) decreases (1)
(iii) solid (1)
60 °C is below the melting point / the melting point is above 60 °C (1)
(b) (i) number of protons in the nucleus of an atom / number of positive charges in the nucleus of an atom
(ii) any suitable, e.g. detecting leaks in pipes / measuring thickness of paper / energy production
(c) (i) 2 (Fe) (1)
3 (NaOH) (1)
(ii) iron(III) oxide loses oxygen / it loses oxygen
Question
The table shows the mass of some of the ions in a 1000 \(cm^3\) sample of sea water.
(a) Answer these questions using only the information in the table.
(i) State which negative ion has the lowest mass in 1000 \(cm^3\) of sea water.
(ii) Give the formulae of the ions in potassium sulfate.
………………………………………………………. and ………………………………………………………
(iii) Calculate the mass of calcium ions in 200cm3
of this sample of sea water.
mass = ………………………… mg
(iv) A sample of this sea water is evaporated.
State the name of the compound which is present in the greatest quantity when this sample is evaporated.
(v) Give the name of the ion which reacts with aqueous silver nitrate to give a cream precipitate.
(b) The \(B_3O_6^{3–}\) ion can be converted to boric acid, \(H_3BO_3\).
Boric acid is also produced when boron trichloride, \(BCl_3\), reacts with water.
Complete the equation for this reaction.
\(BCl_3 + …..H_2O → H_3BO_3 + …..HCl\)
(c) The symbol of a strontium ion is shown.
\(^{87}_{38} Sr^{2+}\)
Deduce the number of electrons, protons and neutrons in one atom of this strontium ion.
number of electrons …………………………………………………………………………………………………….
number of protons ………………………………………………………………………………………………………
number of neutrons …………………………………………………………………………………………………….
(d) Some isotopes of strontium are radioactive.
(i) Give one medical use of radioactive isotopes.
(ii) The isotope \(^{235}\)U is also radioactive.
State the major use of this isotope of uranium.
Answer/Explanation
Answer:
(a) (i) \(B_3O_6^{3-}\) / metaborate
(ii) \(K^+\) AND \(SO_4^{2-}\)
(iii) 80 (mg)
(iv) sodium chloride
(v) bromide
(b) \(3(H_2O)\)
3(HCl)
(c) number of electrons: 36 (1)
number of protons: 38 (1)
number of neutrons: 49 (1)
(d) (i) cancer treatment / tracer (e.g. for thyroid function)
(ii) source of energy / energy production
Question
The diagram shows a blast furnace used in the extraction of iron.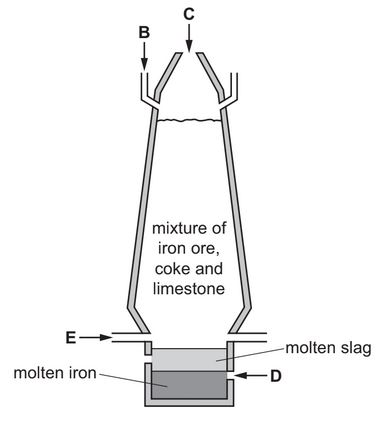
(a) Air is blown into the furnace.
State which letter on the diagram, B, C, D or E, shows where air is blown into the furnace.
(b) (i) Complete the chemical equation for the reduction of iron(III) oxide in the blast furnace.
\(Fe_2O_3 + 3C → ……Fe + ……CO\)
(ii) Explain how this equation shows that iron(III) oxide is reduced.
(c) Calcium carbonate (limestone) is added to the blast furnace.
The calcium carbonate undergoes thermal decomposition.
(i) Complete the word equation for this reaction.
(ii) One of the products of this reaction reacts with impurities in the iron to form slag.
Use the information in the diagram to suggest how you know that molten slag is less dense than molten iron.
(d) (i) Use words from the list to complete these sentences about how steel is made from iron.
acidic basic chlorides methane neutral
nitrogen oxides oxygen sulfates
A gas is blown through the molten iron. The name of this gas is …………………… .
Acidic gases are formed. These acidic gases react with …………………… ……………………
(ii) State one use of mild steel.
(iii) Metals such as chromium are added to iron to make stainless steel.
The symbol for an isotope of chromium is shown.
\(_{24}^{53}Cr\)
Deduce the number of electrons, neutrons and protons in one atom of this isotope of
chromium.
number of electrons ………………………………………………………………………………………………
number of neutrons ………………………………………………………………………………………………
number of protons ………………………………………………………………………………………………..
(e) Chromium conducts electricity and is shiny.
Give two other physical properties of chromium that are characteristic of all metals.
1 ……………………………………………………………………………………………………………………………….
2 ……………………………………………………………………………………………………………………………….
Answer/Explanation
Answer:
(a) E
(b) (i) 2 (Fe) (1)
3 (CO) (1)
(ii) iron oxide loses oxygen / it loses oxygen
(c) (i) calcium oxide (1)
carbon dioxide (1)
(ii) slag floats above the iron
(d) (i) oxygen (1)
basic (1) oxides (1)
(ii) car bodies / machinery
(iii) electrons: 24 (1)
neutrons: 29 (1)
protons: 24 (1)
(e) 1 mark each for any two of:
• conducts heat
• malleable
• ductile