Question
The diagram shows part of the Periodic Table.
Answer the following questions using only the symbols of the elements in the diagram.
Each symbol may be used once, more than once or not at all.
Give the symbol of the element that:
(a) is extracted from bauxite
(b) forms 21% of clean, dry air
(c) forms an oxide which contributes to acid rain
(d) forms an aqueous ion that gives a red-brown precipitate on addition of aqueous
sodium hydroxide
(e) has an atom with a complete outer electron shell.
Answer/Explanation
Answer:
(a) Al
(b) O
(c) N
(d) Fe
(e) Ar
Question
(a) The diagram shows part of the Periodic Table.
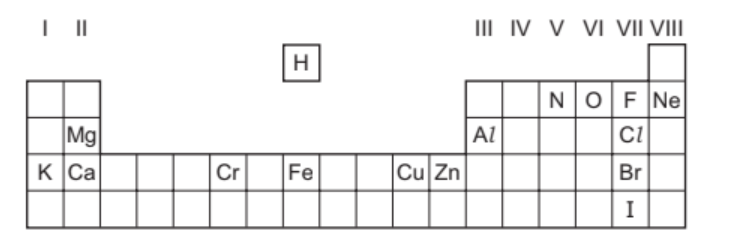
Answer the following questions using only the symbols of the elements in the diagram. Each symbol may be used once, more than once or not at all.
State the symbol of the element that:
(i) is a monoatomic gas at room temperature[1]
(ii) is a liquid at room temperature[1]
(iii) forms a stable ion of type $X^{2-}$[1]
(iv) is extracted from hematite[1]
(v) forms an ion whose aqueous solution gives a grey-green precipitate on addition of aqueous ammonia.[1]
(b) Magnesium has several naturally occurring isotopes.
(i) State the meaning of the term isotopes.[2]
(ii) An isotope of magnesium is shown.
${ }_{12}^{26} \mathrm{Mg}$
Deduce the number of protons and neutrons in this isotope.
number of protons………………………………………………..
number of neutrons………………………………………….[2]
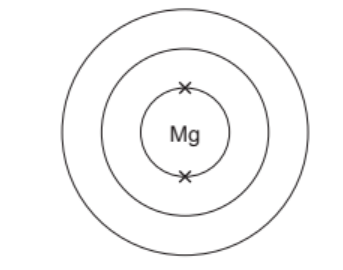
(c) Complete the electronic structure of a magnesium atom.
 [1] [Total: 10]
[1] [Total: 10]
▶️Answer/Explanation
Ans:
(a)(i) Ne / neon
(a)(ii) $\mathrm{Br} /$ Bromine $/ \mathrm{Br}_2$
(a)(iii) O / oxygen
(a)(iv) Fe / iron
(a)(v) $\mathrm{Cr} /$ chromium $/ \mathrm{Cr}^{3 *}$
(b)(i) atoms (1)
(with the) same number of protons but different numbers of neutrons / (with the) same atomic number but different mass
number / (with the) same atomic number but different nucleon number (1)
(b)(ii) protons = 12 (1)
neutrons = 14 (1)
(c) 8 electrons in second shell and 2 electrons in outer shell
Question
The diagram shows part of the Periodic Table.

Answer the following questions using only the elements in the diagram. Each element may be used once, more than once or not at all.
(a) Which element
(i) forms 78% of the air,
……………………………………………………………………………………………………………………… [1]
(ii) has an oxide which is a product of respiration,
……………………………………………………………………………………………………………………… [1]
(iii) is used to make food containers because of its resistance to corrosion,
……………………………………………………………………………………………………………………… [1]
(iv) forms an ion of type \(X^{3+}\),
……………………………………………………………………………………………………………………… [1]
(v) forms an ion whose aqueous solution forms a light blue precipitate on addition of a few drops of aqueous ammonia?
……………………………………………………………………………………………………………………… [1]
(b) Calcium is an element with several naturally-occurring isotopes.
(i) What is the meaning of the term element?
……………………………………………………………………………………………………………………… [1]
(ii) Two of the isotopes of calcium are
\(^{43}_{20}Ca\) and \(^{48}_{20}Ca\)
Complete the table to show the number of protons, neutrons and electrons in one atom of each of these isotopes.

[3]
(iii) Determine the number of electrons in one calcium ion,\( Ca^{2+}\). ……………………………………………………………………………………………………………………… [1]
[Total: 10]
Answer/Explanation
(a)(i) N/ nitrogen;
(a)(ii) C/ carbon/ carbon dioxide;
(a)(iii) Al/ aluminium;
(a)(iv) Cr/Fe/Al/Ti/ chromium/ iron/ aluminium/ titanium;
(a)(v) Cu/ copper
(b)(i) substance containing only one type of atom;
(b)(ii) number of protons: 20 and 20;
number of neutrons: 23 and 28;
number of electrons: 20 and 20;
(b)(iii) 18;