Question
The diagrams show part of the structures of five substances, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ and $\mathbf{E}$.

(a) Answer the following questions about these structures.
Each structure may be used once, more than once or not at al

l.
(i) Which two of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, are covalently bonded? [2]
(ii) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is a diatomic molecule? [1]
(iii) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is a compound? [1]
(iv) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is very soluble in water? [1]
(v) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is used in cutting tools? [1]
(vi) Which one of these structures, $\mathbf{A}, \mathbf{B}, \mathbf{C}, \mathbf{D}$ or $\mathbf{E}$, is used in electrical wiring? [1]
(b) Substance $\mathbf{B}$ is an element.
What is meant by the term element? [1] [Total: 8]
▶️Answer/Explanation
Ans:
(a)(i) B / diamond (1)
D / nitrogen / N2 (1)
(a)(ii) D / nitrogen / N2
(a)(iii) C / lithium chloride / LiCl
(a)(iv) C / lithium chloride / LiCl
(a)(v) B / diamond
(a)(vi) E / copper / Cu
(b) substance in which all the atoms have the same proton number / substance containing (only) one type of atom
Question
Some properties of four substances, A, B, C and D, are shown in the table.
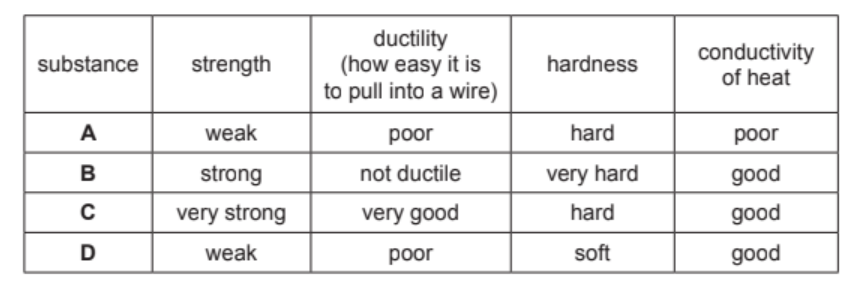
Answer these questions using only the information in the table.
(a) State which substance, A, B, C or D, is best used in the core of an overhead electricity cable.
Explain your answer.
substance ………………………………………………………………………………………………………………….
explanation ………………………………………………………………………………………………………………..
………………………………………………………………………………………………………………………………….
[3]
(b) State which substance, A, B, C or D, is best used for the tip of a drill.
Explain your answer.
substance ………………………………………………………………………………………………………………….
explanation ………………………………………………………………………………………………………………..
…………………………………………………………………………………………………………………………………. [3] [Total: 6]
▶️Answer/Explanation
Ans:
(a) C (1)
very strong (1)
very good ductility / best ductility (1)
(b) B (1)
very hard (1)
strong / good conductor of heat (1)
Question
This question is about metals.
(a) Magnesium is manufactured by the electrolysis of molten magnesium chloride.

(i) What information in the diagram shows that molten magnesium is less dense than molten magnesium chloride?[1]
(ii) One of the products of this electrolysis is magnesium.
State the name of the other product.[1]
An unreactive gas is blown over the surface of the molten magnesium.
(iii) Suggest why an unreactive gas and not air is blown over the surface of the molten magnesium.[1]
(iv) Suggest the name of an unreactive gas which could be used.[1]
(b) The table shows some properties of four metals.

Answer these questions using only the information shown in the table.
(i) Which metal is most suitable to make the body of an aircraft?
Give a reason for your answer.[2]
(ii) Which metal is most suitable to use for overhead power cables?
Give a reason for your answer.[2]
(iii) Which two metals in the table are transition elements?
___ and ___ [1]
(c) Give two properties of transition elements which are not shown by Group I elements.[2]
1
2
(d) Cobalt is added to iron to make steel alloys.
(i) What is meant by the term alloy?[1]
(ii) Give one reason why alloys are used instead of pure metals.[1][Total: 13]
Answer/Explanation
Ans:
(a)(i) magnesium floats (on the molten magnesium chloride)
(a)(ii) chlorine
(a)(iii) to stop the magnesium oxidizing / to stop the magnesium reacting with the air / to stop it oxidizing / to stop it reacting with the air
(a)(iv) argon / krypton / xenon
(b)(i) aluminum
has the lowest density / has a low density
(b)(ii) aluminum
has the best (electrical) conductivity
(b)(iii) cobalt AND nickel
(c) one mark each for any two of:
- high melting points / high boiling points
- high density
- hard / strong
- compounds are coloured
- form ions with different oxidation states
- act as catalysts
(d)(i) mixture of metals / mixture of metal and non-metal / mixture of a metal with another element(1)
(d)(ii) alloy is stronger (than pure metal) / alloy is more resistant to corrosion (than pure metal) / alloy is harder