Question
This question is about iron and iron compounds.
(a) Name the main ore of iron. [1]
(b) In a blast furnace used for the extraction of iron, carbon reacts with oxygen from the air to form carbon monoxide.
Complete the chemical equation for this reaction.
$$
….. \rm{C}+\ldots \rightarrow2 \mathrm{CO}
$$ [2]
(c) In the hotter parts of the furnace, carbon reacts with the iron(III) oxide present in the iron ore.
$$
3 \mathrm{C}+\mathrm{Fe}_2 \mathrm{O}_3 \rightarrow 3 \mathrm{CO}+2 \mathrm{Fe}
$$
How does this equation show that carbon is oxidised? [1]
(d) Limestone is added to the blast furnace. The limestone is converted into calcium oxide and carbon dioxide. The reaction is endothermic.
$$
\mathrm{CaCO}_3 \stackrel{\text { heat }}{\longrightarrow} \mathrm{CaO}+\mathrm{CO}_2
$$
(i) What type of chemical reaction is this? [1]
(ii) What type of oxide is calcium oxide?
Give a reason for your answer. [2]
(e) Iron is a metal.
Give three physical properties that are characteristic of metals.
1……………………………………………
2…………………………………………
3 ……………………………………… [3]
(f) The structure of a compound of iron is shown.

Deduce the molecular formula of this compound to show the number of iron, carbon and oxygen atoms. [1]
▶️Answer/Explanation
Ans:
(a) hematite
(b) 2 (C) (1)
O2 (1)
(c) carbon gains oxygen (from Fe 2O3) / oxygen (from Fe 2O3) combines with carbon
(d)(i) thermal decomposition 1
(d)(ii) basic oxide (1)
calcium is a metal (oxide) (1)
(e) any three from:
• conduct electricity
• conduct heat
• malleable
• ductile
• shiny / lustrous
(f) $Fe_{2}C_{9}O_{9}$
Question
The diagram shows a blast furnace used in the extraction of iron.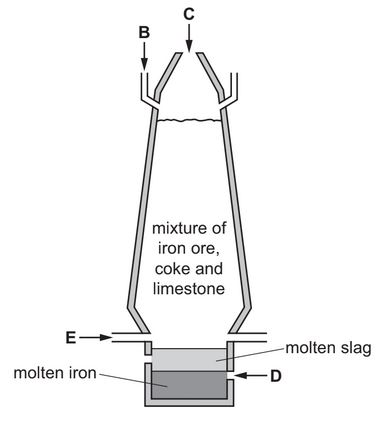
(a) Air is blown into the furnace.
State which letter on the diagram, B, C, D or E, shows where air is blown into the furnace.
(b) (i) Complete the chemical equation for the reduction of iron(III) oxide in the blast furnace.
\(Fe_2O_3 + 3C → ……Fe + ……CO\)
(ii) Explain how this equation shows that iron(III) oxide is reduced.
(c) Calcium carbonate (limestone) is added to the blast furnace.
The calcium carbonate undergoes thermal decomposition.
(i) Complete the word equation for this reaction.
(ii) One of the products of this reaction reacts with impurities in the iron to form slag.
Use the information in the diagram to suggest how you know that molten slag is less dense than molten iron.
(d) (i) Use words from the list to complete these sentences about how steel is made from iron.
acidic basic chlorides methane neutral
nitrogen oxides oxygen sulfates
A gas is blown through the molten iron. The name of this gas is …………………… .
Acidic gases are formed. These acidic gases react with …………………… ……………………
(ii) State one use of mild steel.
(iii) Metals such as chromium are added to iron to make stainless steel.
The symbol for an isotope of chromium is shown.
\(_{24}^{53}Cr\)
Deduce the number of electrons, neutrons and protons in one atom of this isotope of
chromium.
number of electrons ………………………………………………………………………………………………
number of neutrons ………………………………………………………………………………………………
number of protons ………………………………………………………………………………………………..
(e) Chromium conducts electricity and is shiny.
Give two other physical properties of chromium that are characteristic of all metals.
1 ……………………………………………………………………………………………………………………………….
2 ……………………………………………………………………………………………………………………………….
Answer/Explanation
Answer:
(a) E
(b) (i) 2 (Fe) (1)
3 (CO) (1)
(ii) iron oxide loses oxygen / it loses oxygen
(c) (i) calcium oxide (1)
carbon dioxide (1)
(ii) slag floats above the iron
(d) (i) oxygen (1)
basic (1) oxides (1)
(ii) car bodies / machinery
(iii) electrons: 24 (1)
neutrons: 29 (1)
protons: 24 (1)
(e) 1 mark each for any two of:
• conducts heat
• malleable
• ductile
Question
Iron is extracted by heating a mixture of coke (carbon), limestone and iron ore in air in a blast furnace.
A diagram of the blast furnace is shown.
(a) Name the ore of iron added to the blast furnace.
(b) The impurities in the iron ore are removed as slag.
(i) What information in the diagram shows that slag is less dense than molten iron?
(ii) Which one of the substances added to the blast furnace helps to remove the impurities?
Explain how it does this.
substance ……………………………………………………………………………………………………………
explanation ………………………………………………………………………………………………………….
(c) Hot air is blown into the blast furnace.
Explain why.
(d) The chemical equation for one of the reactions in the blast furnace is shown.
\(Fe_2O_3 + 3CO → 2Fe + 3CO_2\)
(i) How does this equation show that \(Fe_2O_3\) has been reduced?
(ii) When 16.0g of \(Fe_2O_3\) react with excess carbon monoxide, 11.2g of iron are produced.
Calculate the mass of iron produced when 4.0g of \(Fe_2O_3\) reactwith excess carbon monoxide.
mass of iron = ………………………… g
(e) An isotope of iron is shown.
\(^{58}_{26} Fe\)
Deduce the number of electrons, protons and neutrons in an atom of this isotope of iron.
number of electrons Ans…………………………………………………………………………………………………….
number of protons ………………………………………………………………………………………………………
number of neutrons …………………………………………………………………………………………………….
(f) Iron is a transition element.
Which two of these statements about iron are correct?
Tick two boxes.
Answer/Explanation
Answer:
(a) hematite
(b) (i) slag floats on iron / slag is above the iron ORA
(ii)
limestone (1)
AND 1 mark each for any 2 of:
• it decomposes / it breaks down
• forms calcium oxide / forms CaO
• reacts with impurities / reacts with silicon dioxide / reacts with silicates / reacts with silica / reacts with \(SiO_2\)
(c) to form carbon monoxide / to burn the coke / to burn the carbon
(d) (i) oxygen removed from \(Fe_2O_3\) / iron oxide loses oxygen
(ii) 2.8 (g)
(e) electrons: 26 (1)
protons: 26 (1)
neutrons: 32 (1)
(f) first box down ticked (iron forms coloured compounds) (1)
second box down ticked (iron can act as a catalyst) (1)