Question
. An alkane molecule of molecular formula \(C_8H_{18}\) undergoes cracking. The equation for the
reaction is shown.
\(C_8H_{18} → Q + 2R\)
Substance R has two carbon atoms per molecule and decolourises aqueous bromine.
What is substance Q?
A butane
B butene
C ethane
D ethene
Answer/Explanation
Ans: A
Question
Gases are separated from liquid air by fractional distillation.
The boiling points of four gases are shown.
Which gas is both monoatomic and a liquid at \(–200^o\) C?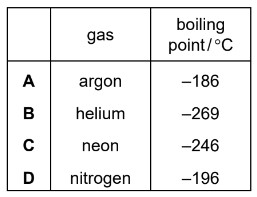
Answer/Explanation
Ans: A
Question
Copper(II) sulfate is prepared by adding excess copper(II) oxide to warm dilute sulfuric acid.
Which purification methods are used to obtain pure solid copper(II) sulfate from the reaction
mixture?
1 crystallisation
2 filtration
3 chromatography
4 distillation
A 1 and 4 B 1 and 2 C 2 and 3 D 3 and 4
Answer/Explanation
Ans: B
Question
A mixture is separated using the apparatus shown.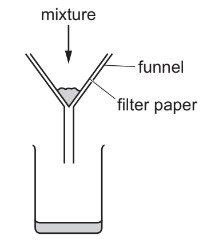
What is the mixture?
A aqueous copper(II) sulfate and aqueous sodium chloride
B aqueous copper(II) sulfate and copper
C copper and sulfur
D ethanol and ethanoic acid
Answer/Explanation
Ans: B