Question
Magnesium oxide has a high melting point.
Carbon dioxide has a low melting point.
Which row identifies the attractive forces that are broken when these compounds are melted?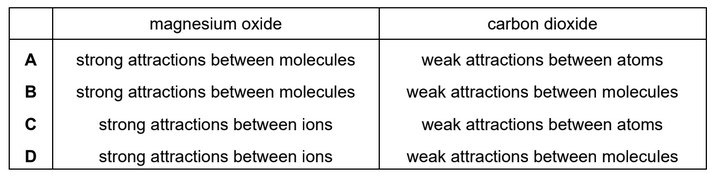
Answer/Explanation
Ans: D
Question
Two elements, P and Q, are in the same period of the Periodic Table.
P and Q react together to form an ionic compound. Part of the lattice of this compound is shown.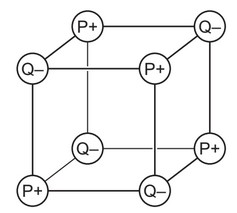
Which statement is correct?
A An ion of P has more electrons than an ion of Q.
B Element P is non-metallic.
C P is to the left of Q in the Periodic Table.
D The formula of the compound is \(P_4Q_4\).
Answer/Explanation
Ans: C
Question
Which statement explains why metals conduct electricity when solid?
A They have atoms which are free to move.
B They have electrons which are free to move.
C They have molecules which are free to move.
D They have positive ions which are free to move.
Answer/Explanation
Ans: B
Question
Which statement describes the structure of an ionic compound?
A. It is a giant lattice of oppositely charged ions.
B. It is a giant lattice of positive ions in a ‘sea’ of electrons.
C. It is a giant molecule of oppositely charged ions.
D. It is a simple molecule of oppositely charged ions.
Answer/Explanation
Ans:
A