Question
The equation for the reaction between gaseous hydrogen and gaseous iodine to form gaseous hydrogen iodide is shown.
H2(g) + I2(g) → 2HI(g)
The reaction is exothermic.
Which statement explains why the reaction is exothermic?
- Energy is released when H–H and I–I bonds are broken.
- The bond energies of the reactants are larger than the bond energies of the products.
- The products are at a higher energy level than the reactants.
- More energy is released when two HI bonds are formed than is used when the H–H and I–I bonds are broken.
Answer/Explanation
Ans:
D
Question
Which row describes the changes that occur in an endothermic reaction?

Answer/Explanation
Ans:
C
Question
Which statement about endothermic and exothermic reactions is correct?
A. In an endothermic reaction, less energy is absorbed in bond breaking than is released in bond forming.
B. In an endothermic reaction, the activation energy is always higher than in an exothermic reaction.
C. In an exothermic reaction, more energy is absorbed in bond breaking than is released in bond forming.
D. In an exothermic reaction, the reactants are higher on an energy level diagram than the products.
Answer/Explanation
Ans:
D
Question
The equation for the complete combustion of methane is shown.
\(CH_4(g) + 2O_2(g) \rightarrow CO_2(g) + 2H_2O(g)\)
The bond energies are shown in the table.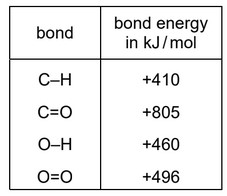
What is the energy change for the reaction?
A –818 kJ /mol B –359 kJ /mol C –323 kJ /mol D +102 kJ / mol
Answer/Explanation
Ans: A