Question
The equation for the manufacture of ammonia in the Haber process is shown.
\(3H_{2}(g) + N_{2}(g)\rightleftharpoons 2NH_{3}(g)\)
The forward reaction is exothermic.
Which row describes the effect of the stated change on the reaction rate and the yield of ammonia?

Answer/Explanation
Ans:
B
Question
How does increasing the concentration affect the reacting particles in a chemical reaction?
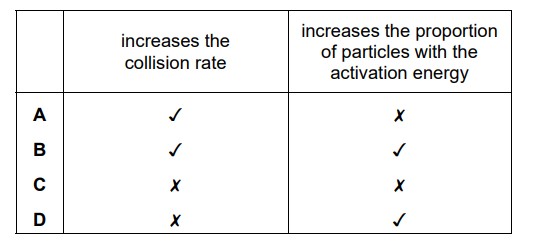
Answer/Explanation
Ans:
A
Question
The apparatus shown is used to measure the rate of a reaction.
Which equation represents a reaction where the rate can be measured using this apparatus?
A \(Mg(s) + 2HCl(aq) \rightarrow MgCl_2(aq) + H_2(g)\)
B \(HCl(aq) + NaOH(aq) \rightarrow NaCl(aq) + H_2O(l)\)
C \(Fe(s) + CuSO_4(aq) \rightarrow Cu(s) + FeSO_4(aq)\)
D \(2Na(s) + Br_2(l) \rightarrow 2NaBr(s)\)
Answer/Explanation
Ans: A
Question
Four statements about the effect of increasing temperature on a reaction are shown.
1 The activation energy becomes lower.
2 The particles move faster.
3 There are more collisions between reacting particles per second.
4 There are more collisions which have energy greater than the activation energy.
Which statements are correct?
A 1, 2 and 3 B 1, 3 and 4 C 2, 3 and 4 D 2 and 3 only
Answer/Explanation
Ans: C