Question
This question is about sodium and compounds of sodium.
(a) (i) Describe the bonding in a metallic element such as sodium.
You may include a diagram as part of your answer.[3]
(ii) Describe how solid sodium conducts electricity.[1]
(b) Some properties of sodium chloride are shown:
-
- melting point of 801°C
- non-conductor of electricity when solid
- conductor of electricity when molten
- soluble in water.
(i) Name the type of bonding in sodium chloride.[1]
(ii) Explain why sodium chloride conducts electricity when molten.[1]
(c) A student determines the concentration of a solution of dilute sulfuric acid, H2SO4, by titration with aqueous sodium hydroxide, NaOH.
step 1 25.0cm3 of 0.200mol/dm3 NaOH is transferred into a conical flask.
step 2 Three drops of methyl orange indicator are added to the conical flask.
step 3 A burette is filled with H2SO4.
step 4 The acid in the burette is added to the conical flask until the indicator changes colour. The volume of acid is recorded. This process is known as titration.
step 5 The titration is repeated several times until a suitable number of results is obtained.
(i) Name the piece of apparatus used to measure exactly 25.0cm3 of 0.200mol/dm3 NaOH in step 1. [1]
(ii) State the colour change of the methyl orange indicator in step 4. from _________ to _________ [1]
(iii) State how the student decides that a suitable number of results have been obtained.[1]
(iv) 20.0cm3 of H2SO4 reacts with 25.0cm3 of 0.200mol/dm3 NaOH.
The equation for the reaction is shown.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Calculate the concentration of H2SO4 using the following steps.
-
-
- Calculate the number of moles in 25.0cm3 of 0.200mol/dm3 NaOH.
-
mol
-
-
- Determine the number of moles of H2SO4 that react with the NaOH.
-
mol
-
-
- Calculate the concentration of H2SO4.
-
mol/dm3
[3] [Total: 12]
Answer/Explanation
Ans:
3(a)(i) positive ions / cations (1)
sea of electrons / mobile electrons / delocalised electrons (1)
attraction between positive ions and electrons (1)
3(a)(ii) electrons move /
electrons mobile /
electrons flow
3(b)(i) ionic
3(b)(ii) ions move /
ions are mobile /
ions flow
3(c)(i) pipette
3(c)(ii) yellow to orange
3(c)(iii) at least two results are within 0.2 cm3 or less
3(c)(iv) 0.005 / 5 x 10–3 (1)
0.0025 / 2.5 x 10–3 (1)
0.125 (1)
Question
(a) Sulfuric acid is made industrially by a four-step process.
step 1 Sulfur is burned in air to produce sulfur dioxide.
step 2 Sulfur dioxide is converted into sulfur trioxide.
step 3 Sulfur trioxide is reacted with concentrated sulfuric acid to produce oleum.
step 4 Oleum is reacted with water to produce concentrated sulfuric acid.
(i) Some sulfur is obtained by mining. Name one other major source of sulfur.
……………………………………………………………………………………………………………………… [1]
(ii) What is the name of the process by which sulfuric acid is made industrially?
……………………………………………………………………………………………………………………… [1]
(iii) Describe the conversion of sulfur dioxide into sulfur trioxide in step 2. In your answer, include:
● a chemical equation for the reaction
● the essential reaction conditions.
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………… [5]
(b) When concentrated sulfuric acid is added to glucose, C6H12O6, a black solid is produced. The concentrated sulfuric acid acts as a dehydrating agent.
(i) What is removed from the glucose in this reaction?
……………………………………………………………………………………………………………………… [1]
(ii) Name the black solid produced in this reaction.
……………………………………………………………………………………………………………………… [1]
(c) The gas hydrogen sulfide, H2S, is produced when concentrated sulfuric acid is added to solid
potassium iodide.
The reaction involves oxidation.
(i) Define the term oxidation in terms of electron transfer.
……………………………………………………………………………………………………………………… [1]
(ii) Complete the dot-and-cross diagram to show the electron arrangement in a molecule of
hydrogen sulfide. Show outer shell electrons only.

[2]
(iii) Hydrogen sulfide has a simple molecular structure. Explain why hydrogen sulfide has a low boiling point.
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………………
……………………………………………………………………………………………………………………… [2]
(d) Dilute sulfuric acid reacts with aqueous sodium hydrogencarbonate in a neutralisation reaction.
\( H_2SO_4(aq) + 2NaHCO_3(aq) \rightarrow Na_2SO_4\)(aq) +\(2H_2O(l) + 2CO_2\)(g)
In a titration, 0.200mol/\(dm^{3}\) aqueous sodium hydrogencarbonate was used to neutralise \(20.0cm^{3}\) of dilute sulfuric acid of concentration 0.150mol/\(dm^{3}\) .
(i) Calculate the number of moles of dilute sulfuric acid used in the titration.
………………………… mol [1]
(ii) Calculate the number of moles of sodium hydrogencarbonate needed to neutralise the dilute sulfuric acid.
………………………… mol [1]
(iii) Calculate the volume, in \(cm^{3}\) , of 0.200mol/\(dm^{3}\) aqueous sodium hydrogencarbonate needed to neutralise the dilute sulfuric acid.
………………………… \(cm^3\) [1] [Total: 17]
Answer/Explanation
4(a)(i) from petroleum or (crude) oil or fossil fuels
4(a)(ii) Contact (process)
4(a)(iii) M1 vanadium pentoxide or vanadium(V) oxide or V2O5 (catalyst);
M2 1–5 atmospheres; (Units required)
M3 450°C; units required
\(M_4 2SO_2 + O_2\rightarrow 2SO_3 \);
M5 equilibrium / reversible reaction in equation or text
4(b)(i) water / \(H_2O\)
4(b)(ii) carbon / C
4(c)(i) (oxidation is) loss of electrons
4(c)(ii) M1 one shared pair between each H and S
M2 four unpaired electrons on S giving S a total of 8 outer shell electrons and no other unpaired electrons
4(c)(iii) M1 weak (attractive) forces OR (attractive) forces need little energy to overcome
M2 forces between molecules / intermolecular
4(d)(i) 0.003
4(d)(ii) 0.006
4(d)(iii) 30
Question
A teacher demonstrated the reactivity of calcium with water. He used the apparatus shown below.
(a) The teacher measured the volume of gas given off at various times during the reaction. He
then repeated the experiment using strontium but keeping all the conditions the same.
The graph obtained from the results is shown below.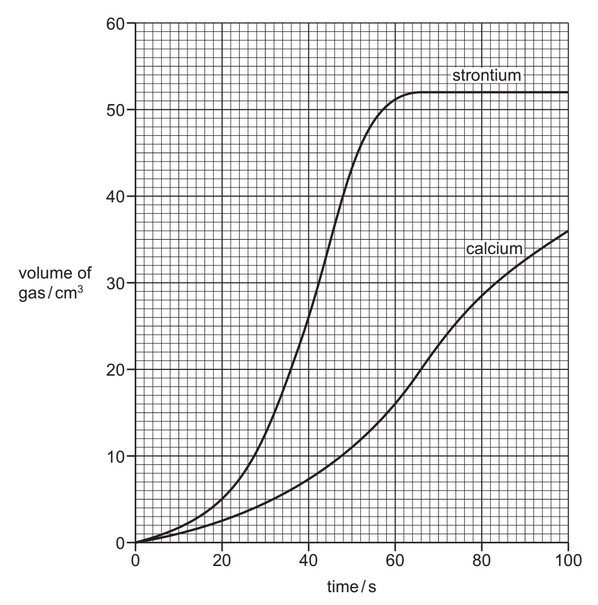
(i) Explain how the graph shows that strontium is more reactive than calcium.
(ii) For the reaction between calcium and water, deduce the volume of gas produced in the
first 50 seconds.
…………….. \(cm^3\)
(iii) At what time was the reaction between strontium and water complete?
…………….. s
(iv) How do you know from the graph that the reaction between calcium and water was not
complete 100 seconds after the reaction started?
(v) Suggest how the rate of reaction changes when the same mass of calcium is used but in
smaller pieces.
(b) The solution formed at the end of the reaction between strontium and water is alkaline. It is a
solution of strontium hydroxide.
The teacher titrated this solution with hydrochloric acid using the apparatus shown below.
(i) What piece of apparatus should be used to put exactly 25.0\(cm^3\) of the strontium hydroxide solution into the flask?
(ii) A few drops of litmus solution was added to the flask.
Explain why litmus is added to the flask and describe what happens to the litmus as the
titration proceeds.
(c) The graph below shows how the pH of the solution in the flask changes as the acid is added.
(i) Describe how the pH of the solution changes as the titration proceeds.
(ii) What volume of acid had been added when the solution had a neutral pH?
(iii) The symbol equation for the reaction is
\(Sr(OH)_2 + 2HCl → SrCl_2 + 2H_2O\)
Give the name of the salt formed in this reaction.
Answer/Explanation
Answer:
(a) (i) gradient/ slope is greater for strontium ora;
(ii) 11(\(cm^3\));
(iii) 64–66(s);
(iv) the line was still going up/the line was still rising;
(v) (rate) increases;
(b) (i) (volumetric) pipette;
(ii)to show end point of titration/ to show when the solution has been neutralised;
litmus goes from blue to pink (at end point);
(c) (i) decreases slowly at first;
then sudden decrease in pH;
then slow decrease;
(ii) 26(\(cm^3\));
(iii) strontium chloride;