Question
Titanium is extracted from an ore called rutile. Rutile is an impure form of titanium(IV) oxide, TiO2.
(a) Rutile is mixed with coke and heated in a furnace through which chlorine gas is passed. The product is gaseous titanium(IV) chloride, TiCl4.
TiO2(s) + 2C(s) + 2Cl2(g) TiCl4(g) + 2CO(g)
The gaseous titanium(IV) chloride produced is condensed into the liquid state. The titanium(IV) chloride is then separated from liquid impurities.
(i) Suggest the name of the process by which liquid titanium(IV) chloride could be separated from the liquid impurities.[1]
(ii) Carbon monoxide, CO(g), is also produced in the reaction.
Why should carbon monoxide not be released into the atmosphere?[1]
(b) Calculate the volume of chlorine gas, Cl2(g), at room temperature and pressure, that reacts completely with 400g of TiO2(s) using the following steps.
TiO2(s) + 2Cl2(g) + 2C(s) TiCl4(g) + 2CO(g)
- Calculate the relative formula mass, Mr, of TiO2.
Mr of TiO2 =
- Calculate the number of moles in 400g of TiO2.
mol
- Determine the number of moles of Cl2 that react with 400g of TiO2.
moles of Cl2 = mol
- Calculate the volume of Cl2 that reacts with 400g of TiO2.
volume of Cl2 = dm3 [4]
(c) Titanium(IV) chloride, TiCl4, is heated with an excess of magnesium, in an atmosphere of argon.
(i) Balance the chemical equation for the reaction.[1]
TiCl4 + ….. Mg Ti + ….. MgCl2
(ii) Titanium(IV) chloride can be reacted with sodium instead of magnesium.
The reaction between titanium(IV) chloride and sodium is similar to the reaction between titanium(IV) chloride and magnesium.
Write a chemical equation for the reaction between titanium(IV) chloride and sodium.[1]
(iii) Suggest why the reaction between titanium(IV) chloride and magnesium is done in an atmosphere of argon and not in air.[1]
(d) After titanium(IV) chloride is heated with magnesium, the unreacted magnesium is removed by adding an excess of dilute hydrochloric acid to the mixture.
The dilute hydrochloric acid also dissolves the magnesium chloride.
The dilute hydrochloric acid does not react with the titanium or dissolve it.
(i) Give two observations and write a chemical equation for the reaction that occurs when dilute hydrochloric acid reacts with magnesium.
1
2
chemical equation [3]
(ii) Name the process that is used to separate the titanium from the mixture after all the magnesium has been removed. [1]
(iii) Titanium does not react with the dilute hydrochloric acid or dissolve in it.
Suggest why titanium does not react with dilute hydrochloric acid.[1]
(e) Magnesium cannot be produced by electrolysis of aqueous magnesium chloride using inert electrodes.
(i) Name the product formed at the negative electrode (cathode) during the electrolysis of aqueous magnesium chloride. [1]
(ii) Suggest how magnesium can be produced from magnesium chloride by electrolysis.[1] [Total: 16]
Answer/Explanation
Ans:
5(a)(i) fractional distillation
5(a)(ii) carbon monoxide is toxic/poisonous
5(b)
- 80
- 5
- 10
- 240
5(c)(i) TiCl4 + 2Mg → Ti + 2MgCl2
5(c)(ii) TiCl4 + 4Na → Ti + 4NaCl
5(c)(iii) magnesium burns in air or oxygen OR reacts with air or oxygen / argon is unreactive or inert
5(d)(i) M1 / 2
bubbles / fizzing / effervescence(1)
M1 / 2 (magnesium or solid) dissolves / disappears / forms solution(1)
M3
Mg + 2HCl → MgCl2 + H2(1)
5(d)(ii) filtration
5(d)(iii) titanium is below hydrogen in the reactivity series ORA
OR titanium less reactive than hydrogen ORA
OR titanium coated with an oxide layer
5(e)(i) hydrogen
5(e)(ii) Heat until magnesium chloride is molten and electrolyze
Question
Substances can be classified as elements, compounds or mixtures.
(a) What is meant by the term compound?[2]
(b) Mixtures can be separated by physical processes.
A sequence of physical processes can be used to separate common salt (sodium chloride) from a mixture containing sand and common salt only.
Give the order and the correct scientific term for the physical processes used to separate the common salt from the mixture.[4]
1
2
3
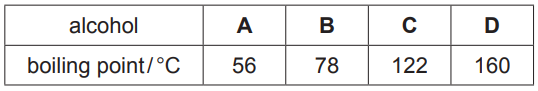
The boiling points of four different alcohols, A, B, C and D, are shown.
(c) A student suggested that the apparatus shown could be used to separate the mixture of alcohols.
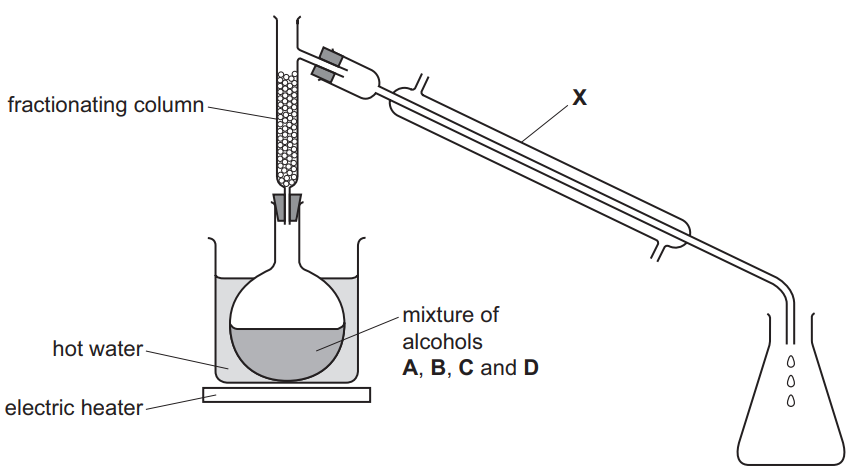
(i) Apparatus X needs to have cold water flowing through it.[2]
-
- Draw an arrow on the diagram to show where the cold water enters apparatus X.
- Name apparatus X.
(ii) Part of the fractionating column is missing. This means that the experiment will not work.[2]
-
- Draw on the diagram the part of the fractionating column which is missing.
- Explain why the experiment will not work with this part of the fractionating column missing.
(iii) Suggest why a Bunsen burner is not used to heat the flask.[1]
(iv) A hot water bath cannot be used to separate alcohols C and D.
Explain why.[2] [Total: 13]
Answer/Explanation
Ans:
(a) a substance made from two (or more) elements
chemically combined
(b) dissolving
filtration
evaporation / crystallization
three correct stages in the correct order
(c)(i) condenser
arrow pointing into lower aperture only
(c)(ii) stopper shown in diagram
gases or vapours escape
(c)(iii) (mixture is) (in)flammable
(c)(iv) water bath cannot exceed 100 (°C)
C AND D have a boiling point above 100 (°C)
Question
The table gives some information about five substances.

(a) Which substance in the table has ionic bonding? ……………………………………………………………………………………………………………………………. [1]
(b) Which substance in the table has a giant covalent structure? ……………………………………………………………………………………………………………………………. [1]
(c) Name a method you could use to separate a mixture of substance J and water. ……………………………………………………………………………………………………………………………. [1]
(d) Name a method you could use to obtain substance F from a mixture of substance F and water.
(e) Describe how you could obtain a solid sample of substance H from a mixture of substance H and substance
(f) Substance J is a metal. Describe how substance J is able to conduct electricity when it is a solid.
▶️Answer/Explanation
Ans:
1(a) H
1(b) G
1(c) filtration
1(d) fractional
distillation
1(e) add / mix / stir/ dissolve / shake /heat with water
filter/decant
heat (filtrate) or (leave filtrate to) evaporate
1(f) electrons
(electrons) move / flow (throughout structure)