Question
(a) Atoms are made of protons, neutrons and electrons. Atoms of the same element are known as isotopes.
(i) Complete the table.[2]
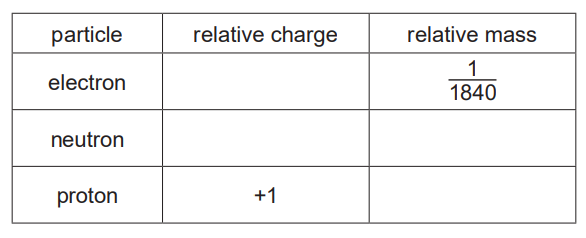
(ii) \(_{12}^{24}\textrm{Mg}\) and \(_{12}^{25}\textrm{Mg}\) are isotopes of magnesium.
Complete the table to show the numbers of electrons, neutrons and protons in these isotopes of magnesium.[2]

(iii) Explain why magnesium ions have a charge of 2+.[1]
(b) Mg2+ ions have the electronic structure 2,8.
Give the formula of the following particles which have the same electronic structure as Mg2+ ions.
-
- a cation (positive ion)
- an anion (negative ion)
- an atom
[3] [Total: 8]
Answer/Explanation
Ans:
2(a)(i)
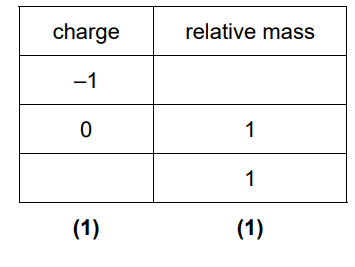
Mark by column
2(a)(ii)

Mark by row
2(a)(iii) (they have) 2 more protons than electrons
OR
(they have) 2 fewer electrons than protons
OR
(they have) 12 protons and 10 electrons
2(b) Na+ or Al 3+ (1)
F– or O2– or N3– (1)
Ne (1)
Question
Complete the table to:
● deduce the number of protons, electrons and neutrons in the magnesium atom and copper ion
shown
● identify the atom or ion represented by the final row.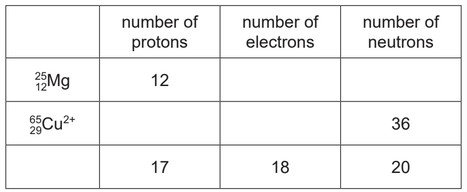
Answer/Explanation
Answer:
Mg: 12 and 13 (1)
\(Cu^{2+}: 29\) and 27 (1)
37(above)
and17(below) (1)
Cl (1)
1– (1)
Question
The table shows the numbers of protons, neutrons and electrons in particles A to I.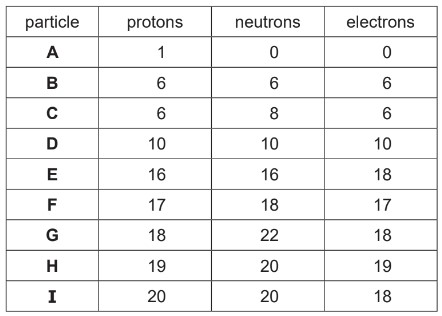
Answer the following questions about particles A to I. Each letter may be used once, more than
once or not at all.
(a) State which of the particles A to I:
(i) is an anion
(ii) are cations
(iii) are noble gas atoms
(iv) is a halogen atom
(v) is a Group i atom
(vi) have the same nucleon number
(vii) causes acidity in aqueous solutions
(viii) is used to define the relative atomic mass of elements
(b) Explain why B and C are isotopes of the same element.
Answer/Explanation
Answer:
(a) (i) E
(ii) A
I
(iii) D
G
(iv) F
(v) H
(v)(i) G and I
(ii) A
(iii) B
(b) same proton number
different neutron number