Question
This question is about sodium and compounds of sodium.
(a) (i) Describe the bonding in a metallic element such as sodium.
You may include a diagram as part of your answer.[3]
(ii) Describe how solid sodium conducts electricity.[1]
(b) Some properties of sodium chloride are shown:
-
- melting point of 801°C
- non-conductor of electricity when solid
- conductor of electricity when molten
- soluble in water.
(i) Name the type of bonding in sodium chloride.[1]
(ii) Explain why sodium chloride conducts electricity when molten.[1]
(c) A student determines the concentration of a solution of dilute sulfuric acid, H2SO4, by titration with aqueous sodium hydroxide, NaOH.
step 1 25.0cm3 of 0.200mol/dm3 NaOH is transferred into a conical flask.
step 2 Three drops of methyl orange indicator are added to the conical flask.
step 3 A burette is filled with H2SO4.
step 4 The acid in the burette is added to the conical flask until the indicator changes colour. The volume of acid is recorded. This process is known as titration.
step 5 The titration is repeated several times until a suitable number of results is obtained.
(i) Name the piece of apparatus used to measure exactly 25.0cm3 of 0.200mol/dm3 NaOH in step 1. [1]
(ii) State the colour change of the methyl orange indicator in step 4. from _________ to _________ [1]
(iii) State how the student decides that a suitable number of results have been obtained.[1]
(iv) 20.0cm3 of H2SO4 reacts with 25.0cm3 of 0.200mol/dm3 NaOH.
The equation for the reaction is shown.
H2SO4 + 2NaOH → Na2SO4 + 2H2O
Calculate the concentration of H2SO4 using the following steps.
-
-
- Calculate the number of moles in 25.0cm3 of 0.200mol/dm3 NaOH.
-
mol
-
-
- Determine the number of moles of H2SO4 that react with the NaOH.
-
mol
-
-
- Calculate the concentration of H2SO4.
-
mol/dm3
[3] [Total: 12]
Answer/Explanation
Ans:
3(a)(i) positive ions / cations (1)
sea of electrons / mobile electrons / delocalised electrons (1)
attraction between positive ions and electrons (1)
3(a)(ii) electrons move /
electrons mobile /
electrons flow
3(b)(i) ionic
3(b)(ii) ions move /
ions are mobile /
ions flow
3(c)(i) pipette
3(c)(ii) yellow to orange
3(c)(iii) at least two results are within 0.2 cm3 or less
3(c)(iv) 0.005 / 5 x 10–3 (1)
0.0025 / 2.5 x 10–3 (1)
0.125 (1)
Question
(a) Propanol is a solvent.
Sugar is soluble in propanol. Salt (sodium chloride) is insoluble in propanol.
A student wants to separate a mixture of solid salt and solid sugar.
(i) Describe how she could separate the salt from the sugar.
You may draw a labelled diagram to help you answer this question.[3]
(ii) Describe how the student could obtain solid sodium chloride from a solution of sodium chloride in water.[1]
(b) The diagram shows the structure of sodium chloride.
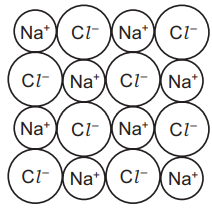
(i) Deduce the simplest formula for sodium chloride.[1]
(ii) What type of bonding is present in sodium chloride?
Put a ring around the correct answer.[1]
covalent ionic metallic weak
(c) The diagram shows the apparatus used to electrolyze a concentrated aqueous solution of sodium chloride.

(i) Which letter on the diagram, A, B, C or D, represents the electrolyte?[1]
(ii) Name the product formed at[2]
the positive electrode,
the negative electrode. [Total: 9]
Answer/Explanation
Ans:
(a) (i) Any three of:
- add propanol to the mixture and shake (or stir)
- implication of filtration of solution/diagram of filter funnel and filter paper
REJECT: diagram of filter paper circle on top of funnel
- sugar solution goes through the filter paper/ sugar solution is the filtrate/ diagram shows sugar solution (labelled) passing through filter paper
- salt or sodium chloride remains on filter paper/ diagram shows salt or sodium chloride (labelled) remaining on filter paper
(ii) evaporate the water/evaporation
IGNORE: heat
ALLOW: distillation
(b) (i) NaCl
ALLOW: Na+Cl–
REJECT: Na++ Cl– / multiples, e.g. 2NaCl
(ii) ionic
(c) (i) D
(ii) positive electrode → chlorine/Cl2
IGNORE: Cl
negative electrode → hydrogen/H2
IGNORE: H
IF: correct electrode products reversed = 1 mark
Question
The table shows some properties of four substances, A, B, C and D.
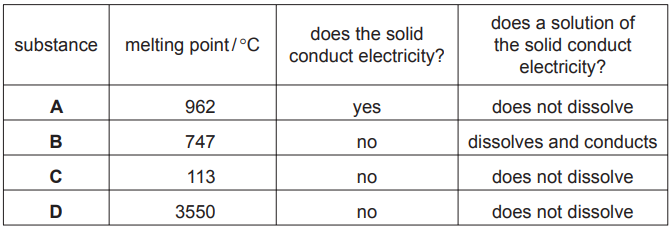
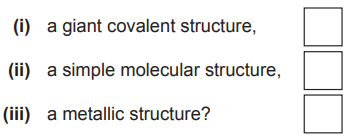
(a) Which one of these substances has[3]
(b) A student carried out an experiment to determine the rate of reaction of calcium carbonate with excess hydrochloric acid.
CaCO3(s) + 2HCl(aq) → CaCl 2(aq) + CO2(g) + H2O(l)
He recorded the loss of mass of the reaction mixture over a period of time.

(i) Explain why the reaction mixture decreases in mass.[1]
He carried out the reaction at constant temperature using 2 g of calcium carbonate in small pieces. The hydrochloric acid was in excess.
He plotted his results on a grid. This is shown below.
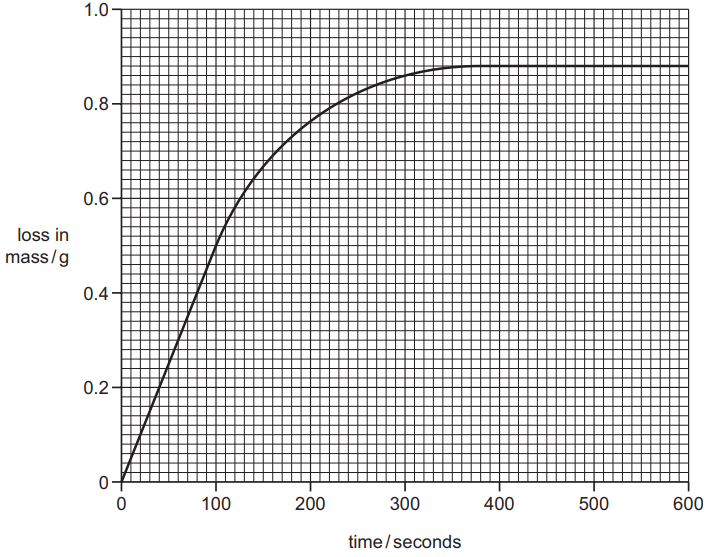
(ii) At what time has the reaction just finished?
__ s [1]
(iii) From the graph, deduce the loss in mass in the fi rst 100 seconds.
__ g [1]
(iv) The student repeated the experiment keeping everything the same except for the size of the pieces of calcium carbonate. He used smaller pieces of calcium carbonate but the mass used was the same.
On the grid above, draw a line to show how the loss of mass changes with time when smaller pieces of calcium carbonate are used. [2]
(v) State the effect of increasing the concentration of hydrochloric acid on the rate (speed) of this reaction when all other factors remain constant.[1] [Total: 9]
Answer/Explanation
Ans:
(a) (i) D
(ii) C
(iii) A
(b) (i) loss of carbon dioxide/ loss of gas
(ii) accept values from 360–380
ALLOW: 6 min to 6min 20s / 6⅓ min
(iii) 0.5(g)
(iv) (initial) gradient greater/ slope greater and starts at 0, 0;
same final volume
(v) (rate) increases
IGNORE: more carbon dioxide per second
ALLOW : (rate) faster