Question
Ethanoic acid is manufactured by the reaction of methanol with carbon monoxide.
An equilibrium mixture is produced.
\(CH_{3}OH\left ( g \right )+CO\left ( g \right )\rightleftharpoons CH_{3}COOH\left ( g \right )\)
(a) State two characteristics of an equilibrium.[2]
1
2
(b) The purpose of the industrial process is to produce a high yield of ethanoic acid at a high rate of reaction.
The manufacture is carried out at a temperature of 300°C.
The forward reaction is exothermic.
Use this information to state why the manufacture is not carried out at temperatures:[2]
-
- below 300°C
- above 300°C.
(c) Complete the table using only the words increases, decreases or no change.[3]

(d) Suggest which of the following metals is a suitable catalyst for the reaction. Give a reason for your answer.[2]
aluminium calcium cobalt magnesium potassium
suitable catalyst
reason
(e) Ethanoic acid is a member of the homologous series of carboxylic acids.
State the general formula of this homologous series.[1]
(f) Draw the structure of the carboxylic acid containing three carbon atoms. Show all of the atoms and all of the bonds. [2]
(g) When carboxylic acids react with alcohols, esters are produced.
The formula of ester X is CH3CH2CH2COOCH3.
(i) Name ester X. [1]
(ii) Give the name of the carboxylic acid and the alcohol that react together to produce ester X.[2]
carboxylic acid
alcohol
(h) Ester Y has the following composition by mass:
C, 48.65%; H, 8.11%; O, 43.24%.
Calculate the empirical formula of ester Y.
empirical formula = [3]
(i) Ester Z has the empirical formula C2H4O and a relative molecular mass of 88.
Determine the molecular formula of ester Z.
molecular formula = [1] [Total: 19]
Answer/Explanation
Ans:
5(a) the rate of forward reaction equals the rate of the reverse reaction (1)
concentrations of reactants and products are constant (1)
5(b) reaction too slow (1)
yield of ethanoic acid too low (1)
5(c)

5(d) cobalt (1)
transition element (1)
5(e) CnH2n+1COOH
5(f) COOH fully displayed (1)

whole molecule completely correct (1)
5(g)(i) methyl butanoate
5(g)(ii) butanoic acid (1)
methanol (1)
5(h) C 48.65 / 12
H 8.11 / 1
O 43.24 / 16
OR
4.05:8.11:2.70 (1)
fractions shown dividing all by smallest
OR
1.5:3:1
OR
3:6:2 (1)
C3H6O2 (1)
5(i) C4H8O2
Question
Dinitrogen tetroxide, \(N_2O_4\), decomposes into nitrogen dioxide, \(NO_2\). The reaction is reversible.
A gas syringe containing a mixture of dinitrogen tetroxide and nitrogen dioxide gases was sealed
and heated. After reaching equilibrium the mixture was a pale brown colour.
(a) State what is meant by the term equilibrium.
(b) The plunger of the gas syringe is pushed in. The temperature does not change. The mixture
initially turns darker brown. After a few seconds the mixture turns lighter brown because the
equilibrium shifts to the left.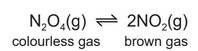
(i) Explain why the mixture initially turns darker brown.
(ii) Explain why the position of equilibrium shifts to the left.
(c) The forward reaction is endothermic.
(i) State what happens to the position of equilibrium when the temperature of the mixture is
increased.
(ii) State what happens to the rate of the forward reaction and the rate of the backward
reaction when the temperature of the mixture is increased.
rate of the forward reaction …………………………………………………………………………………….
rate of the backward reaction …………………………………………………………………………………
Answer/Explanation
Answer:
(a) the rate of forward reaction equals the rate of the reverse reaction (1)
concentrations of reactants and products are constant (1)
(b) (i) (increased pressure) nitrogen dioxide particles or molecules (forced) closer together
OR
same number of nitrogen dioxide particles or molecules in a smaller volume
(ii) fewer number of gas moles or molecules on left hand side or reactant side (of the equation) ORA
(c) (i) shifts to the right
(ii) increase / faster (1)
increase / faster (1)
Question
Chalcopyrite, \(FeCuS_2\), is used in the manufacture of sulfuric acid in the Contact process.
(a) In the first stage of the process, chalcopyrite reacts with oxygen in the air to produce
sulfur dioxide, \(SO_2\), iron(III) oxide and copper(II) oxide.
Complete the chemical equation for the reaction of \(FeCuS_2\) with oxygen.
\(4FeCuS_2 + 13O_2\) → …………….. + …………….. + ……………..
(b) Sulfur dioxide is then converted to sulfur trioxide.
\(2SO_2 + O_2 \leftrightarrow 2SO_3\)
The reaction is exothermic. It is also an equilibrium.
(i) State two features of an equilibrium.
1 …………………………………………………………………………………………………………………………
2 …………………………………………………………………………………………………………………………
(ii) State the temperature and pressure used in this reaction.
Include units.
● temperature …………………………………………………………………………………………………..
● pressure ………………………………………………………………………………………………………..
(iii) Name the catalyst used.
(iv) Explain why a catalyst is used.
(v) Describe and explain, in terms of equilibrium, what happens when the temperature is
increased.
(c) Concentrated sulfuric acid is a dehydrating agent.
When glucose is dehydrated, carbon and one other product are formed.
Complete the equation to show the dehydration of glucose, \(C_6H_{12}O_6\).
\(C_6H_{12}O_6\) → ………..C + …………………
Answer/Explanation
Answer:
(a) \((4FeCuS 13O_2) → 2Fe_2O_3 + 4CuO + 8SO_2\)
\(Fe_2O_3\) and CuO as a product (1)
Equation fully correct (1)
(b) (i) M1 rate of forward reaction = rate of reverse reaction (1)
M2 concentration of reactants and products are constant (1)
(ii) M1 450 °C (1)
M2 1-2 atm (1)
(iii) vanadium(V) oxide
(iv) increase rate of reaction
(v) M1 equilibrium shifts to left hand side (1)
M2 forward reaction is exothermic (1)
(c) \(C_6H_{12}O_6 → 6C + 6H_2O\)
\(H_2O\) (1)
balance (1)