Question
Carbon-12 is a stable isotope of carbon. Its nuclide notation is shown in Fig. 11.1.
Carbon-14 is an unstable isotope of carbon. Its nuclide notation is shown in Fig. 11.2.

(a) Determine the numbers of electrons, protons and neutrons in an atom of carbon-12 and the
numbers of electrons, protons and neutrons in an atom of carbon-14.
Complete Table 11.1.
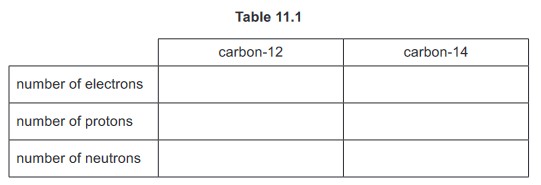
(b) Fig. 11.3 shows the decay curve for a sample of carbon-14.
Use the graph to determine the half-life of carbon-14.
half-life = ……………………………………….. years
Answer/Explanation
Answer:
(a) 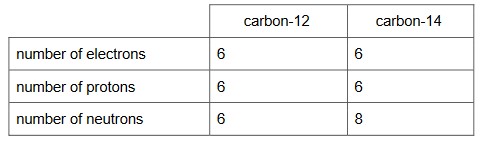
(b) any indication on graph of line from 8000
5600 (years)
Question
A nucleus of americium-241 has the nuclide notation shown.
\(^{241}_{95}\) Am
(a) (i) Determine the number of neutrons in a nucleus of americium-241.
number of neutrons =…………………………………………………………………………………………….
(ii) Determine the charge on a nucleus of americium-241.
charge =………………………………………………………………………………………………….
(b) Americium-241 decays by emitting α-particles.
Put a tick in the box next to each correct statement.

(c) Americium-241 has a half-life of 432 years.A sample contains 16 mg of americium-241.Calculate the time it takes until only 4.0 mg of americium-241 are left in the sample.
time = ………………………………………. years
Answer/Explanation
Answer:
(a)
(i) 146
(ii) positive
95
(b) tick in 3rd box
tick in 4th box
(c) idea of 2 half-lives
(432 × 2) = 864 (years)
