Question
The isotope americium-241 is represented by
\(_{95}^{241}\textrm{Am}\)
This isotope decays by an α-emission to an isotope of neptunium (Np).
(a) Complete the nuclide equation for this decay.[3]
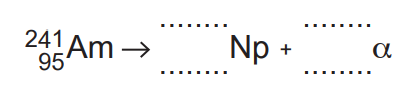
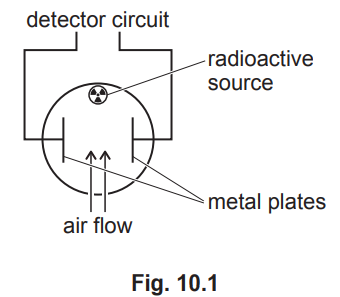
(b) Fig. 10.1 shows a simple diagram of a smoke detector. The smoke detector contains a small sample of americium-241. This isotope ionises the air between the metal plates in the detector.
(ii) Suggest and explain two reasons why smoke detectors use an isotope that emits α-particles rather than an isotope that emits γ-radiation.
1.
2. [2] [Total: 8]
Answer/Explanation
Ans:
(a) \(_{95}^{241}\textrm{Am}\rightarrow _{93}^{237}\textrm{Np}+_{2}^{4}\propto \)
237Np nucleon number correct for Np
93Np proton number correct for Np
\(+_{2}^{4}\propto \) alpha notation correct
(b)(i) alpha (particles emitted from americium)
move close to / hit molecules in the air (between the metal plates)
removing electrons (out of the molecules)
(b)(ii) Any two from:
-
-
- alpha not penetrating / short range AND alpha (particles) stopped by smoke particles
- alpha (particles) more highly ionising (than gamma) AND ionise air more easily
- range of alpha particles is short / alpha is not penetrating AND alpha less harmful (to humans)
-
Question
(a) (i) One isotope of iridium-194 is represented by
\(^{194}_{77}Ir\)
This isotope decays by β-emission to a stable isotope of platinum (Pt).
Complete the nuclide equation for this decay.
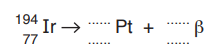
(ii) The half-life of iridium-194 is 19 hours. A sample of iridium-194 has an initial count-rate of 1100 counts / min.
Calculate the count-rate from this sample after 38 hours.
count-rate =………………………………………………………………………………………………………………………
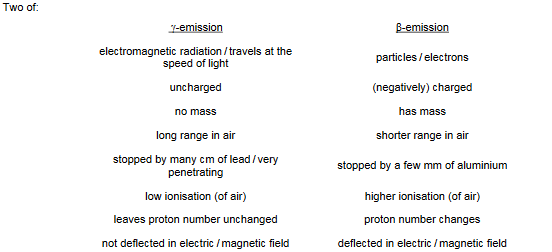
(b) State two ways in which γ-emission differs from β-emission.
1 ……………………………………………………………………………………………………………………………..
2 ……………………………………………………………………………………………………………………………..
Answer/Explanation
Answer:
(a)(i) Nucleon number for Pt: 194
Proton number for Pt: 78
Symbol for beta particle: \(^0_{-1}β\)
(ii) After 1 half-life / 19 hrs, count rate = 1100 / 2 = 550 counts / min
After 2 half-lives / 38 hrs, count rate = 550 / 2 = 275 counts / min
OR
38 hrs = 2 half-lives
After 38 hrs / 2 half-lives, count rate = 1100 / 4 = 275 counts / min
(b)