Question
Some radioactive nuclei decay to give new nuclei which are also radioactive. Part of a series of decays is shown.
$$
{ }_{92}^{238} \mathrm{U} \rightarrow{ }_{90}^{234} \mathrm{Th} \rightarrow{ }_{91}^{234} \mathrm{~Pa} \rightarrow{ }_{92}^{234} \mathrm{U} \rightarrow{ }_{90}^{230} \mathrm{Th} \rightarrow{ }_{88}^{226} \mathrm{Ra}
$$
How many decays involve the emission of a -particle?
A 1
B 2
C 3
D 5
Answer/Explanation
Ans: B
Question
Why are some radioactive sources stored in boxes made from lead?
A Lead absorbs emissions from the radioactive sources.
B Lead decreases the half-life of radioactive sources.
C Lead increases the half-life of radioactive sources.
D Lead repels emissions from the radioactive sources.
Answer/Explanation
Ans:A
Question
A radioactive isotope of carbon 14C decays by beta emission to give an isotope of nitrogen 14N and a beta particle. The equation for the reaction is shown.
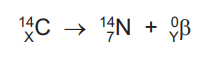
What is the value of X and of Y?
X | Y | |
A | 6 | –1 |
B | 6 | 1 |
C | 8 | –1 |
D | 8 | 1 |
Answer/Explanation
- Ans: A
Question
The radioactive isotope of hydrogen undergoes beta decay to the isotope 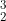 He .
He .
What is the nuclide notation for the hydrogen isotope?
A 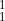 H
H
B 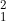 H
H
C 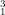 H
H
D 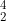 H
H
Answer/Explanation
Ans: C
Question
The chemical symbol for sodium is Na. The equation represents the radioactive decay of sodium-24.
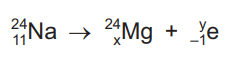
What are the numbers x and y?
| x | y |
A | 10 | 0 |
B | 10 | 1 |
C | 12 | 0 |
D | 12 | 1 |
Answer/Explanation
Ans: C
