Question
(a) State the proton number, nucleon number and the value of the charge on an α-particle.proton number
nucleon number …………………………………………………………………………………………………………..
charge ………………………………………………………………………………………………………………………….
[3]
(b) A nucleus of strontium-90 consists of 38 protons and 52 neutrons. Strontium-90 is radioactive
and decays by β-emission to an isotope of yttrium. The symbol for strontium is Sr and the symbol
for yttrium is Y. Write down the nuclide equation of this decay.
[3]
(c) The half-life of radon-220 is 56 s. A sample of radon-220 is in a container. After 112 s the mass of radon-220 is 9.2 mg.
Calculate the mass of the original sample.
mass =…………………………………………………….. [2]
[Total: 8]
Answer/Explanation
Ans:
(a) 2 ,4 ,+2
(b)
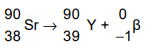
nucleon numbers 90 on both sides of equation
Sr and proton number 38 on left AND Y and proton number 39 on right

(c)
(original mass = 4 / 9.2 =) 37 mg
2 half-lives
