Question
In an experiment, smoke particles are suspended in air and viewed through a microscope.
The smoke particles move about with short random movements.
Which of the following statements is correct?
Air particles have large masses compared to smoke particles and they move in one direction only.
Air particles have large masses compared to smoke particles and they move in random directions.
Air particles move at high speeds compared to smoke particles and they move in one direction only.
Air particles move at high speeds compared to smoke particles and they move in random directions.
Answer/Explanation
Ans: D
Question
Very small pollen grains are suspended in water. A bright light shines from the side.
When looked at through a microscope, small specks of light are seen to be moving in a random, jerky manner.
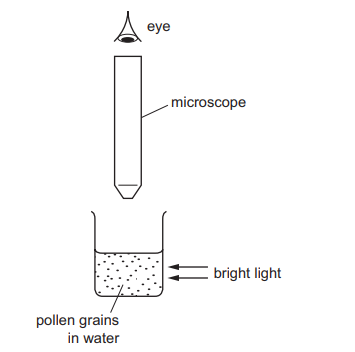
What are the moving specks of light?
pollen grains being hit by other pollen grains
pollen grains being hit by water molecules
water molecules being hit by other water molecules
water molecules being hit by pollen grains
Answer/Explanation
Ans: B
Question
Why can a gas be compressed easily into a smaller volume?
The molecules are far apart.
The molecules do not attract each other.
The molecules move randomly.
The volume of each molecule can be reduced.
Answer/Explanation
Ans: A
Question
The molecules of a substance in a particular state of matter move freely with random motion. The average speed of the molecules is increasing.
What is being described?
A a gas being heated
B a liquid evaporating
C a solid being heated
D a solid melting
Answer/Explanation
Ans: A
Question
Air is trapped in a cylinder by a piston. The piston is pushed inwards and the volume of the air is reduced.
The temperature of the trapped air remains constant.
Which row describes how the average speed of the air molecules and the average distance between them changes?
| average speed of molecules | average distance between molecules |
A | increases | decreases |
B | increases | unchanged |
C | unchanged | decreases |
D | unchanged | increases |
Answer/Explanation
Ans: C
Because the volume has decreased, the particles will collide more frequently with the walls of the container
