Question
The thermal capacity of object Y is greater than that of object Z. What is a consequence of this?
Object Y needs less thermal energy to melt it than object Z.
Object Y needs less thermal energy to raise its temperature by 1
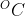 than object Z.
than object Z.Object Y needs more thermal energy to melt it than object Z.
Object Y needs more thermal energy to raise its temperature by 1
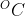 than object Z.
than object Z.
Answer/Explanation
Ans: D
Question
A night storage heater contains a large block of material that is heated electrically during the night. During the
day the block cools down, releasing thermal energy into the room.

Which thermal capacity and which night-time temperature increase will cause the most energy to
be stored by the block?
| thermal capacity of block | night-time temperature increase |
A | large | large |
B | large | small |
C | small | large |
D | small | small |
Answer/Explanation
Ans: A
Question
Four different metal blocks are given the same quantity of thermal energy.
Which block has the greatest thermal capacity?
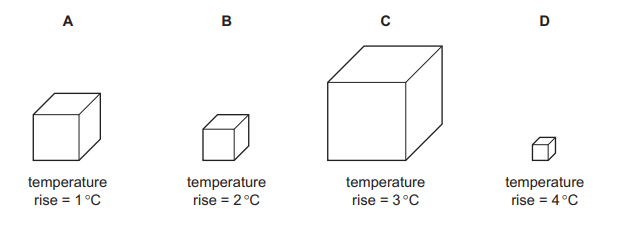
Answer/Explanation
Ans: A
Question
When a hot gas is left to cool, its internal energy decreases.
What causes this?
a decrease in the kinetic energy of the gas particles
a decrease in the gravitational potential energy of the gas particles
an increase in the average speed of the gas particles
an increase in the average distance of separation of the gas particles.
Answer/Explanation
Ans: A
Question
Which statement defines the thermal capacity (heat capacity) of a solid body?
the energy needed to melt the body without a change in temperature
the energy needed to raise the temperature of the body by one degree Celsius
the increase in the volume of the body when its temperature is raised by one degree Celsius
the total amount of internal energy in the body
Answer/Explanation
Ans: B
