Question
Three students are describing the structure of an atom.
student 1 All the positively charged particles are in the nucleus.
student 2 Positive electrons are in the nucleus.
student 3 Negative electrons orbit around the nucleus.
Which students are making a correct statement?
A 1, 2 and 3
B 1 and 2 only
C 1 and 3 only
D 2 and 3 only
Answer/Explanation
Ans: C
Question
The nuclide notation of the isotope strontium-90 is 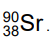
Which statement is correct?
A A nucleus of strontium-90 has 38 neutrons.
B A nucleus of strontium-90 has 52 neutrons.
C A nucleus of strontium-90 has 90 electrons.
D A nucleus of strontium-90 has 90 neutrons.
Answer/Explanation
Ans: B
Question
What are isotopes of an element?
A atoms of a different element with a different number of neutrons
B atoms of a different element with a different number of protons
C atoms of the same element with a different number of neutrons
D atoms of the same element with a different number of protons
Answer/Explanation
Ans: C
Question
Four nuclides are represented below.
Which pair of nuclides are isotopes of the same element?
A E and G
B E and L
C G and L
D G and M
Answer/Explanation
Ans: A
Question
The symbol for a radioactive nuclide of carbon is ![]()
How many neutrons are in its nucleus?
A 6 B 8 C 14 D 20
Answer/Explanation
Ans: B
