Question
Discuss molecular formula provide?
A. The simplest ratio of atoms in a molecule
B. The actual numbers of atoms in a molecule
C. The number of molecules in one mole
D. The types of bonds in a molecule
▶️Answer/Explanation
Markscheme : B
A molecule is comprised of two or more atoms that have been chemically combined. A molecular formula is a chemical formula of a molecular compound that shows the kinds and numbers of atoms present in a molecule of the compound.
Question
A student heated a known mass of zinc powder in an open crucible until there was no further mass change and recorded the final mass.
What would the student be able to derive from this data?
I. Percentage composition of zinc oxide
II. Empirical formula of zinc oxide
III. Molecular formula of zinc oxide
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
Markscheme: A
The student would be able to derive the following information:
I. Percentage composition of zinc oxide – Yes, by comparing the initial and final masses, the student can determine the percentage of zinc in the zinc oxide formed.
II. Empirical formula of zinc oxide – Yes, by determining the moles of zinc and oxygen involved in the reaction, the student can find the empirical formula.
III. Molecular formula of zinc oxide – No, the molecular formula cannot be determined from this experiment alone. To find the molecular formula, additional information about the molar mass of the compound is needed.
Therefore, the correct answer is A. I and II only.
Question
Calculate molar mass of a gas according to the following experimental data?
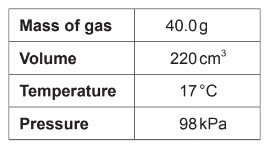
Ideal gas constant = 8.31 \(JK^{-1} mol^{-1}\)
A. \(\frac{40.0 \times 8.31 \times 290}{98 \times 0.220}\)
B. \(\frac{98 \times 0.220}{40.0 \times 8.31 \times 290}\)
C. \(\frac{40.0 \times 8.31 \times 17}{98 \times 0.220}\)
D. \(\frac{98 \times 220}{40.0 \times 8.31 \times 17}\)
▶️Answer/Explanation
Markscheme: A
To find the molar mass of the gas, you can rearrange the ideal gas law equation \(\mathrm{PV}=\mathrm{nRT}\) to solve for the number of moles (\(\mathrm{n}\)) and then use the formula for molar mass (\(\mathrm{M}\)):
\[\mathrm{Molar\ mass} (\mathrm{M}) = \frac{\text{Mass of gas}}{\text{Number of moles}}\]
First, find the number of moles using the ideal gas law:
\[\mathrm{n} = \frac{\mathrm{PV}}{\mathrm{RT}}\]
Now, substitute the given values:
\[\mathrm{n} = \frac{(98 \, \mathrm{kPa}) \times (0.220 \, \mathrm{L})}{(8.31 \, \mathrm{J} \, \mathrm{K}^{-1} \, \mathrm{mol}^{-1}) \times (290 \, \mathrm{K})}\]
Now, substitute the value of \(\mathrm{n}\) into the formula for molar mass:
\[\mathrm{M} = \frac{\text{Mass of gas}}{\text{Number of moles}} = \frac{40.0 \, \mathrm{g}}{\mathrm{n}}\]
so, \[\mathrm{M} =\frac{40.0 \times 8.31 \times 290}{98 \times 0.220}\]
Question
$0.20 \mathrm{~mol}$ of magnesium is mixed with $0.10 \mathrm{~mol}$ of hydrochloric acid.
$$
\mathrm{Mg}(\mathrm{s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{H}_2(\mathrm{~g})
$$
Which is correct?
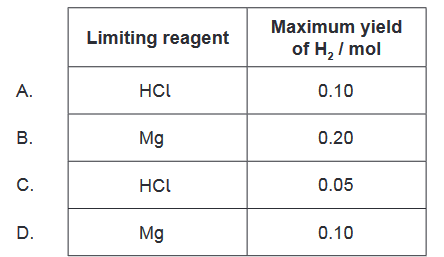
▶️Answer/Explanation
Solution:
To determine the limiting reagent, we need to compare the amount of moles of each reactant and their stoichiometric ratio in the balanced chemical equation.
From the equation, we can see that 1 mole of $\mathrm{Mg}$ reacts with 2 moles of $\mathrm{HCl}$ to produce 1 mole of $\mathrm{H} _2$.
For Mg: $0.20 \mathrm{~mol}$
For $\mathrm{HCl}: 0.10 \mathrm{~mol}$
So, if all the $\mathrm{HCl}$ reacts, it will require $0.05 \mathrm{~mol}$ of $\mathrm{Mg}(0.10 \mathrm{~mol}~~ \mathrm{HCl} / 2 \mathrm{~mol} ~~\mathrm{HCl}$ per $1 \mathrm{~mol}$ $\mathrm{Mg}=0.05 \mathrm{~mol}~~ \mathrm{Mg})$
Since we have more $\mathrm{Mg}$ than the required amount $(0.20 \mathrm{~mol}>0.05 \mathrm{~mol}), \mathrm{Mg}$ is not the limiting reagent. Therefore, $\mathrm{HCl}$ is the limiting reagent.
The maximum yield of $\mathrm{H} _2$ will be determined by the limiting reagent, which is $\mathrm{HCl}$ in this case.
The balanced chemical equation shows that 2 moles of $\mathrm{HCl}$ are required to produce 1 mole of $\mathrm{H}_ 2$. The amount of $\mathrm{HCl}$ available is $0.10 \mathrm{~mol}$, which means that the maximum amount of $\mathrm{H} _2$ that can be produced is:
$0.10 \mathrm{~mol} ~\mathrm{HCl} \times\frac{1 \mathrm{~mol}~ \mathrm{H}_ 2 }{ 2 \mathrm{~mol}~ \mathrm{HCl}}=0.05 \mathrm{~mol} ~\mathrm{H}_ 2$
Therefore, the correct answer is $\mathrm{C}$, which shows that the limiting reagent is $\mathrm{HCl}$, and the maximum yield of $\mathrm{H} _2$ is 0.05 mol.
Question
Which amount, in mol, of sodium chloride is needed to make $250 \mathrm{~cm}^3$ of $0.10 \mathrm{~mol}^{-3}$ solution?
A. $4.0 \times 10^{-4}$
B. $0.025$
C. $0.40$
D. $25$
▶️Answer/Explanation
Solution:
To calculate the amount of sodium chloride needed to make a $0.10 \mathrm{~mol} / \mathrm{L}$ solution in $250 \mathrm{~mL}$ of solvent, we can use the formula:
moles of solute $=$ concentration $x$ volume
where concentration is in $\mathrm{mol} / \mathrm{L}$, and volume is in $\mathrm{L}$. Since the volume is given in $\mathrm{cm}^3$, we need to convert it to $L$ by dividing it by 1000 :
moles of solute $=0.10 \mathrm{~mol} / \mathrm{L} \times 250 \mathrm{~cm}^3 / 1000=0.025 \mathrm{~mol}$
Therefore, the correct answer is $B$, which shows that 0.025 mol of sodium chloride is needed to make a $0.10 \mathrm{~mol} / \mathrm{L}$ solution in $250 \mathrm{~cm}^3$ of solvent.