Question
Discuss mass of one molecule of \(C_{60}\) ?
A. \(1.0 \times 10^{-22}\)
B. \(2.0 \times 10^{-23}\) g
C. \(8.3 \times 10^{-24}\) g
D. \(1.2 \times 10^{-21}\) g
▶️Answer/Explanation
Markscheme: D
The molecular mass of a substance in grams is equal to its molar mass, which is numerically equal to the molecular weight (mass) of one mole of the substance.
The molecular formula for \(C_{60}\) (Buckminsterfullerene, also known as a buckyball) represents a molecule with 60 carbon atoms. The molar mass of \(C_{60}\) can be calculated by adding the atomic masses of 60 carbon atoms.
The atomic mass of carbon (\(C\)) is approximately 12.01 g/mol.
\[ \text{Molar mass of } C_{60} = 60 \times \text{Atomic mass of } C \]
\[ \text{Molar mass of } C_{60} = 60 \times 12.01 \, \text{g/mol} \]
\[ \text{Molar mass of } C_{60} = 720.6 \, \text{g/mol} \]
Now, to find the mass of one molecule of \(C_{60}\), we need to divide this molar mass by Avogadro’s number (\(N_A\)).
\[ \text{Mass of one molecule of } C_{60} = \frac{\text{Molar mass}}{N_A} \]
\[ \text{Mass of one molecule of } C_{60} = \frac{720.6 \, \text{g/mol}}{6.0 \times 10^{23} \, \text{mol}^{-1}} \]
\[ \text{Mass of one molecule of } C_{60} \approx 1.2 \times 10^{-21} \, \text{g} \]
Question
20\(cm^3\) of gas A reacts with 20\(cm^3\) of gas B to produce 10\(cm^3\) of gas \(A_xB_y\) and 10\(cm_3\) of excess gas A.
Calculate correct values for subscripts x and y in the empirical formula of the product \(A_xB_y\) (g)?
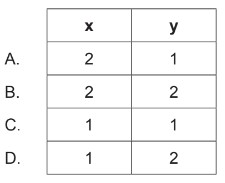
▶️Answer/Explanation
Markscheme: D
Let’s analyze the information provided:
1. \(20 \mathrm{~cm}^3\) of gas A reacts with \(20 \mathrm{~cm}^3\) of gas B.
2. The reaction produces \(10 \mathrm{~cm}^3\) of gas \(A_xB_y\) and \(10 \mathrm{~cm}^3\) of excess gas A.
From this information, we can deduce the following:
The initial moles of A and B are equal since the volumes are the same.
The reaction consumes all of B, as there is no excess of B mentioned.
\(10 \mathrm{~cm}^3\) of excess gas A remains unreacted.
Given that excess gas A is \(10 \mathrm{~cm}^3\), and \(10 \mathrm{~cm}^3\) of gas \(A_xB_y\) is produced, it implies that \(A_xB_y\) is formed by the combination of \(A\) and \(B\), and the remaining \(10 \mathrm{~cm}^3\) of A is in excess.
Since \(A_xB_y\) is formed from 20 \(\mathrm{cm}^3\) of A and 20 \(\mathrm{cm}^3\) of B, and the excess A is 10 \(\mathrm{cm}^3\), it suggests that the composition of \(A_xB_y\) is 1 part A and 2 parts B.
Therefore, the correct values for subscripts \(x\) and \(y\) in the empirical formula of the product \(A_xB_y\) are: \[ A_1B_2 \]
Question
Which best explains why complexes of d-block elements are coloured?
A. Light is absorbed when electrons are promoted between $d$ orbitals.
B. Light is emitted when electrons are promoted between $d$ orbitals.
C. Light is absorbed when electrons return to lower energy $d$ orbitals.
D. Light is emitted when electrons return to lower energy $d$ orbitals.
▶️Answer/Explanation
Solution:
The $d-$block elements have incompletely filled d orbitals, and these orbitals are relatively close in energy. When visible light falls on the complex, the electrons in the d orbitals can be excited to higher energy levels, which are usually antibonding molecular orbitals. These excited electrons create a color because the absorbed light is complementary to the color we see. The color of the complex depends on the energy difference between the d orbitals, which in turn depends on the nature of the ligands and the oxidation state of the central metal ion.
Question
Which elements are considered to be metalloids?
- Gallium
- Germanium
- Arsenic
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
▶️Answer/Explanation
Solution:
Metalloids are elements that have properties of both metals and nonmetals. They are intermediate in their properties between metals and nonmetals, and have characteristics of both. In the periodic table, the metalloids are located along the zigzag line that separates metals from nonmetals.
Germanium and arsenic are two of the five elements that are commonly recognized as metalloids. Germanium (Ge) is a lustrous, grayish-white metalloid with an atomic number of 32. It is used as a semiconductor in electronics, and also as an alloying agent in some types of steel. Germanium has both metallic and nonmetallic properties, such as being a good semiconductor, but also being brittle and lacking ductility.
Arsenic (As) is a metalloid that has a silvery-gray appearance and an atomic number of 33. It is used in some pesticides and herbicides, and in alloys with other metals. Arsenic has both metallic and nonmetallic properties, such as being a good conductor of electricity, but also being toxic and brittle.
Gallium (Ga), on the other hand, is a metallic element with an atomic number of 31. It has a silvery appearance and is soft enough to be cut with a knife. It is used in some semiconductors and LEDs. Although gallium shares some properties with metalloids, it is not considered a metalloid.
$\colorbox{yellow}{ Correct Option: C}$
Question
Which property of elements increases down a group but decreases across a period?
A. Atomic radius
B. Electronegativity
C. Ionic radius
D. Ionization energy
▶️Answer/Explanation
Solution:
Atomic radius is the distance from the nucleus to the outermost electron shell of an atom. Down a group in the periodic table, the number of electron shells increases as you move from one element to the next. This means that the atomic radius of the elements increases down a group.
Across a period in the periodic table, the number of electron shells remains the same while the number of protons in the nucleus increases. This causes the electrons to be pulled in more tightly to the nucleus, resulting in a smaller atomic radius as you move from left to right across the period.
Electronegativity (B) is the tendency of an atom to attract electrons towards itself when it is part of a compound. Ionization energy (D) is the energy required to remove an electron from an atom, while ionic radius (C) is the radius of an ion. These properties do not follow the trend described in the question.
$\colorbox{yellow}{ Correct Option: A}$