Question
What are the numbers of neutrons and electrons in \(^{32}_{16}S^{2-}\)?

▶️Answer/Explanation
>Markscheme: B
The symbol \(^{32}_{16}\rm S^{2-}\) represents the sulfur ion with a charge of -2. The superscript (top number) indicates the mass number, the subscript (bottom number) indicates the atomic number, and the charge is indicated as a superscript on the upper right side.
For a neutral sulfur atom (\(^{32}_{16}\rm S\)), the number of electrons is equal to the atomic number, which is 16.
Since the ion has a charge of -2, it means that there are two more electrons than the number of protons. Therefore, for \(^{32}_{16}\rm S^{2-}\), there are \(16 + 2 = 18\) electrons.
To find the number of neutrons, you can subtract the number of protons (which is equal to the atomic number) from the mass number. For \(^{32}_{16}S\), there are \(32 – 16 = 16\) neutrons.
So, the numbers of neutrons and electrons in \(^{32}_{16}\rm S^{2-}\) are 16 neutrons and 18 electrons.
Question
What is the correct labelling of the blocks of the periodic table?

▶️Answer/Explanation
Markscheme: D

The periodic table is traditionally divided into four blocks based on the electron configurations of the elements. These blocks are labeled as follows:
1. s-block: The first two groups (Group 1 and Group 2, including helium) are part of the s-block. Elements in the s-block have their outermost electrons in s-orbitals.
2. p-block: Groups 13 to 18 make up the p-block. Elements in the p-block have their outermost electrons in p-orbitals.
3. d-block: The transition metals, which constitute Groups 3 to 12, form the d-block. Elements in the d-block have their outermost electrons in d-orbitals.
4. f-block: The inner transition metals, which include the lanthanides and actinides, form the f-block. These are placed separately at the bottom of the periodic table. The f-block is often further divided into the lanthanides (rare earth elements) and the actinides. The electronic configurations of these elements involve the filling of f-orbitals.
These labels are based on the way electrons are added to atomic orbitals as you move across a period and down a group in the periodic table. The s, p, d, and f designations correspond to different types of atomic orbitals.
Question
Three elements, $\mathrm{X}, \mathrm{Y}$, and $\mathrm{Z}$ are in the same period of the periodic table. The relative sizes of their atoms are represented by the diagram.
Which general trends are correct?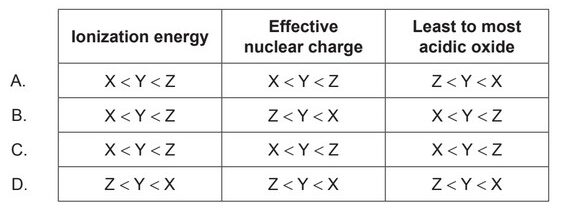
Answer/Explanation
Solution:
The correct general trends are:
- $\colorbox{yellow}{Ionization energy: $\mathrm{X}<\mathrm{Y}<\mathrm{Z}$}$
- $\colorbox{yellow}{Effective nuclear charge: $\mathrm{X}<\mathrm{Y}<\mathrm{Z}$}$
- $\colorbox{yellow}{Least to most acidic oxide: $\mathrm{X}<\mathrm{Y}<\mathrm{Z}$}$
$\colorbox{yellow}{ Correct Option: C}$
Explanation: In a period of the periodic table, the atomic radius decreases from left to right due to an increase in effective nuclear charge, which attracts the electrons more strongly, and a constant shielding effect. Therefore, since $\mathrm{X}, \mathrm{Y}$, and $\mathrm{Z}$ are in the same period and have decreasing radii, $\mathrm{X}$ has the largest atomic radius, $\mathrm{Y}$ has an intermediate radius, and $\mathrm{Z}$ has the smallest radius.
Ionization energy is the energy required to remove an electron from an atom or ion in the gas phase. As the atomic radius decreases, the ionization energy generally increases due to the stronger attraction between the positively charged nucleus and the negatively charged electrons. Therefore, since $\mathrm{X}$ has the largest atomic radius, it will have the lowest ionization energy, and $\mathrm{Z}$ with the smallest atomic radius will have the highest ionization energy.
Effective nuclear charge is the net positive charge experienced by an electron in an atom or ion. As the atomic radius decreases, the effective nuclear charge generally increases due to the increased attraction between the nucleus and the electrons. Therefore, since $\mathrm{X}$ has the largest atomic radius, it will experience the smallest effective nuclear charge, and $\mathrm{Z}$ with the smallest atomic radius will experience the highest effective nuclear charge.
Acidic oxides are oxides that react with water to form acidic solutions. Generally, oxides of nonmetals are acidic, while oxides of metals are basic. As we move across a period from left to right, the metallic character decreases, and the nonmetallic character increases. Therefore, the basicity of the oxides decreases from left to right. Since $\mathrm{X}$ is the most metallic, its oxide will be the most basic, while $\mathrm{Z}$ is the most nonmetallic, and its oxide will be the most acidic.
Question
Which statement best describes the intramolecular bonding in $\mathrm{HCN}(\mathrm{l})$ ?
A. Electrostatic attractions between $\mathrm{H}^{+}$and $\mathrm{CN}^{-}$ ions
B. Hydrogen bonding
C. Van der Waals forces and hydrogen bonding
D. Electrostatic attractions between pairs of electrons and positively charged nuclei
Answer/Explanation
Solution:
The statement that best describes the intramolecular bonding in $\mathrm{HCN}(\mathrm{l})$ is:
$\colorbox{yellow}{(D). Electrostatic attractions between pairs of electrons and positively charged nuclei.}$
Explanation:
Intramolecular bonding refers to the chemical bonds that hold atoms together within a molecule.
In $\mathrm{HCN}$, the intramolecular bonding is primarily due to the sharing of electrons between the atoms. The carbon atom shares a pair of electrons with the nitrogen atom to form a covalent bond, and the hydrogen atom shares a pair of electrons with the carbon atom to form another covalent bond.
The covalent bonds in $\mathrm{HCN}$ are formed by the electrostatic attractions between pairs of electrons and positively charged nuclei. The electrons are negatively charged and are attracted to the positively charged nuclei of the atoms they are bonded to.
In option A, the statement describes the bonding in an ionic compound, where positively charged and negatively charged ions are held together by electrostatic attractions.
In option B, hydrogen bonding occurs between molecules that have hydrogen atoms bonded to highly electronegative atoms (such as nitrogen or oxygen) that have lone pairs of electrons. $\mathrm{HCN}$ does not contain any highly electronegative atoms with lone pairs, so it cannot form hydrogen bonds.
In option C, van der Waals forces are weak intermolecular forces that occur between all atoms and molecules, but they do not play a significant role in intramolecular bonding in $\mathrm{HCN}$.
Question
What is the name of the compound with formula $\mathrm{Ti}_3\left(\mathrm{PO}_4\right)_2$ ?
A. Titanium phosphate
B. Titanium(II) phosphate
C. Titanium(III) phosphate
D. Titanium(IV) phosphate
Answer/Explanation
Solution:
The name of the compound with formula $\mathrm{Ti}_3\left(\mathrm{PO}_4\right)_2$ is:
$\colorbox{yellow}{(B). Titanium(II) phosphate.}$
Explanation:
To name this compound, we first need to determine the oxidation state of titanium. In this case, we can use the formula of the compound to find the total charge on the compound, which is zero.
The oxidation state of phosphate ion is $-3$, and there are two phosphate ions in the formula, which gives a total charge of $-6$. Therefore, the total charge on the three titanium ions must be $+6$ to balance the negative charge on the phosphate ions.
Since there are three titanium ions, each titanium ion must have an oxidation state of $+2$ to give a total of $+6$. Therefore, the name of the compound is titanium(II) phosphate.
Note that the Roman numeral in the name indicates the oxidation state of titanium, which is determined from the formula of the compound.