Question
Analytical and spectroscopic techniques enable chemists to identify and determine structures
of compounds.
(a) An unknown organic compound, X, comprising of only carbon, hydrogen and oxygen was found to contain 48.6% of carbon and 43.2% of oxygen.
Determine the empirical formula.
▶️Answer/Explanation
Ans:
To determine the empirical formula of the unknown organic compound \(X\), we need to find the simplest whole number ratio of atoms of each element in the compound.
Given:
Percentage of carbon (C) in compound \(X\) = 48.6%
Percentage of oxygen (O) in compound \(X\) = 43.2%
To find the percentage of hydrogen (H) in compound \(X\), we can use the fact that the percentages of all elements in a compound must add up to 100%. Therefore, the percentage of hydrogen can be calculated as follows:
\[ \text{Percentage of hydrogen} = 100\% – (\text{Percentage of carbon} + \text{Percentage of oxygen}) \]
\[ \text{Percentage of hydrogen} = 100\% – (48.6\% + 43.2\%) \]
\[ \text{Percentage of hydrogen} = 8.2\% \]
Now, we can convert these percentages to masses (assume a total mass of 100 g for ease of calculation):
Mass of carbon (C) = \(48.6\% \times 100 \, \text{g} = 48.6 \, \text{g}\)
Mass of hydrogen (H) = \(8.2\% \times 100 \, \text{g} = 8.2 \, \text{g}\)
Mass of oxygen (O) = \(43.2\% \times 100 \, \text{g} = 43.2 \, \text{g}\)
Next, we need to determine the moles of each element in the compound using their respective molar masses:
Molar mass of carbon (C) = \(12.01 \, \text{g/mol}\)
Molar mass of hydrogen (H) = \(1.008 \, \text{g/mol}\)
Molar mass of oxygen (O) = \(16.00 \, \text{g/mol}\)
Now, we can calculate the moles of each element:
Moles of carbon (C) = \(\frac{48.6 \, \text{g}}{12.01 \, \text{g/mol}} \approx 4.05 \, \text{mol}\)
Moles of hydrogen (H) = \(\frac{8.2 \, \text{g}}{1.008 \, \text{g/mol}} \approx 8.13 \, \text{mol}\)
Moles of oxygen (O) = \(\frac{43.2 \, \text{g}}{16.00 \, \text{g/mol}} \approx 2.70 \, \text{mol}\)
Now, we find the simplest whole number ratio of moles of each element by dividing each by the smallest number of moles (which is approximately 2.70 mol for oxygen):
Ratio of moles of carbon (C) = \(\frac{4.05 \, \text{mol}}{2.70 \, \text{mol}} \approx 1.50\)
Ratio of moles of hydrogen (H) = \(\frac{8.13 \, \text{mol}}{2.70 \, \text{mol}} \approx 3.01\)
Ratio of moles of oxygen (O) = \(\frac{2.70 \, \text{mol}}{2.70 \, \text{mol}} = 1.00\)
These ratios represent the subscripts of each element in the empirical formula. To obtain whole numbers, we can multiply each ratio by a common factor. In this case, the smallest ratio is 1.00 for oxygen, so we can multiply each ratio by 2 to obtain whole numbers:
Ratio of carbon (C) = 1.50 × 2 ≈ 3
Ratio of hydrogen (H) = 3.01 × 2 ≈ 6
Ratio of oxygen (O) = 1.00 × 2 = 2
Therefore, the empirical formula of the unknown organic compound \(X\) is \(C_3H_6O_2\).
Question
The mass spectrum of X is shown.
(b) Identify fragments responsible for the peaks at m/z 74 and 45 using section 28 of the data booklet.
m/z 74:
m/z 45:
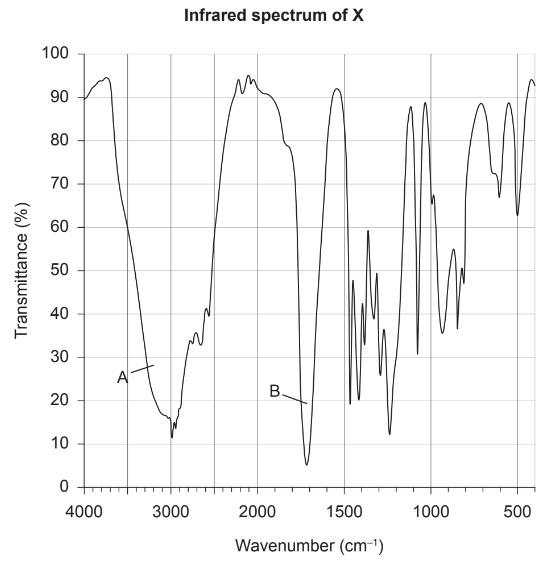
The infrared spectrum of X is shown.
▶️Answer/Explanation
Ans:
m/z 74: molecular ion / \(M^+\) / \(C_3H_6O_2^+\)
m/z 45: \(COOH^+ / C_2H_5O^+\)
Question
(c) Deduce the structural formula of X.
▶️Answer/Explanation
Ans:
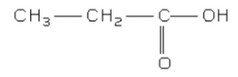
Question
(d) Identify the bonds making the major contribution to peaks A and B using section 26 of the data booklet.
A:
B:
▶️Answer/Explanation
Ans:
A: O-H in carboxylic acids
B: C=O