| Polymers NEET and AIIMS Special |
| Polymers Refresher Course |
| Polymers : Master File |
| Polymers : Quick Revision Notes |
| Polymers : Concepts File |
| Polymers : Brain Map |
| Polymers Reference Book |
About this unit
Classification- Natural and synthetic, methods of polymerization (addition and condensation), copolymerization. Some important polymers: natural and synthetic like polyesters, bakelite; rubber, Biodegradable and non-biodegradable polymers.
POLYMERS
The macromolecules formed by the union between same or different molecules.
Polymerisation : The process of joining together the simple molecules is known as polymerisation.
Monomers : Small molecules which are joined together to form polymers are known as monomers.
Degree of polymerisation : The number of monomolecules(n) which combine to form a given macromolecule is called the degree of polymerisation.
High polymers : Such polymers have high degree of polymerisation.
Oligomers : They have low degree of polymerisation.
Distinction between polymers and macromolecules : Polymers have repeat units and a macromolecule may or may not have repeat units eg chlorophyll and polythene.
Homopolymers : Polymers containing one type of monomer units.
Copolymers : Polymers containing two or more types of monomer units.
CLASSIFICATION OF POLYMERS
They may be divided into two categories.
- Natural polymers – They are obtained from natural sources eg poly saccharides (starch, cellulose), Proteins (polymers of amino acids), gums, resins (cross linked polymers formed by compounds containing double or triple bonds slowly oxidised by atmospheric oxygen). natural rubber (polymer of isoprene), Nucleic acids (polymers of nucleotides) silk and wool (polymers of amino acids).
- Synthetic polymers – Polymers prepared by synthesis (man made), are known as synthetic polymers eg. polystyrene, nylon, PVC etc.
CLASSIFICATION BASED ON STRUCTURE
- Linear polymers – Polymers containing the monomeric units linked together to form long straight chains stacked over one another to give packed structure.
Such polymers have high tensile strength, high densities, high m.p. and b.p. Examples – fibres and plastics.
- Branched polymers – Long chain of monomer units containing side chains of different length forms branched polymers. The chains are loosely packed, hence polymers have low density, low m.p. and low tensile strength. Examples – Amylopectin and glycogen.
- Cross-linked or three dimensional polymers – Such polymers have three dimensional network and are hard, brittle and rigid. Examples – Bakelite, melamine.
STEREOCHEMICAL CLASSIFICATION
Polymers of propylene can be classified into
- Isotactic – with all methyl groups on one side of extended chain
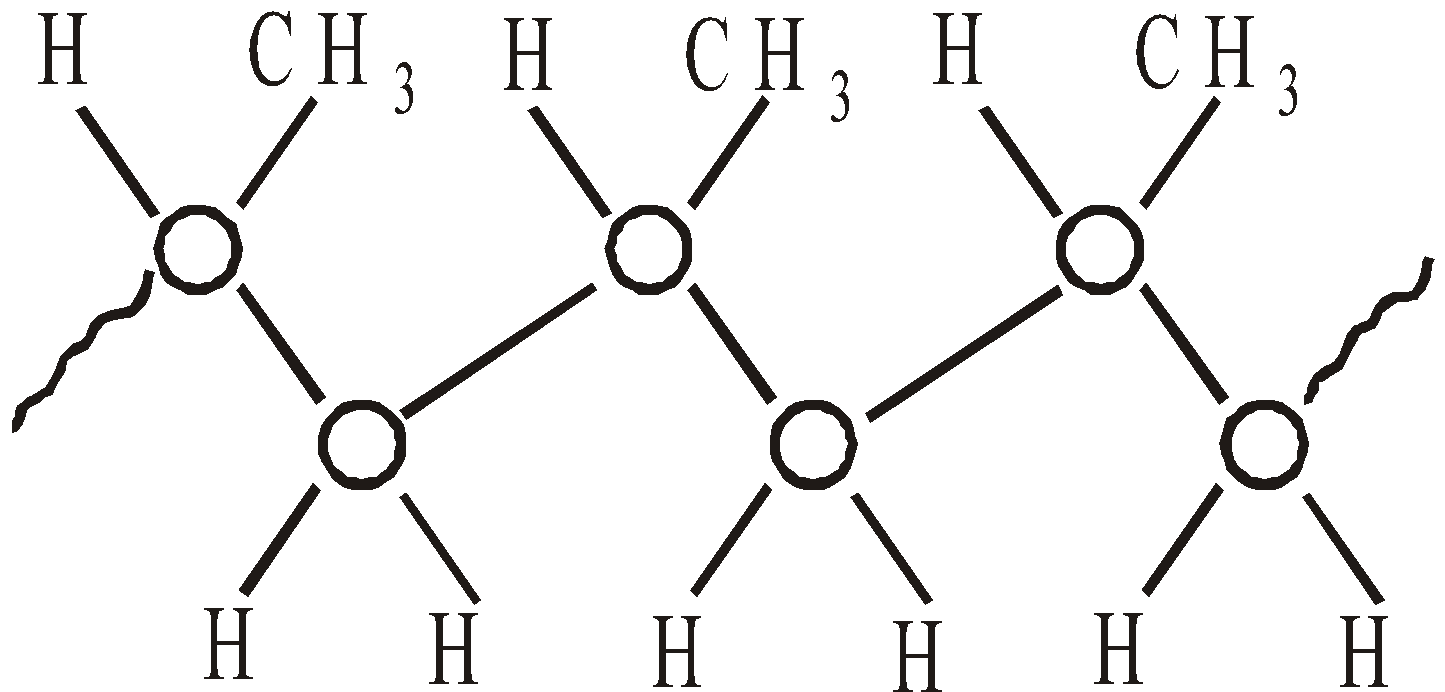
- Syndiotactic – with methyl groups alternating regularity side to side
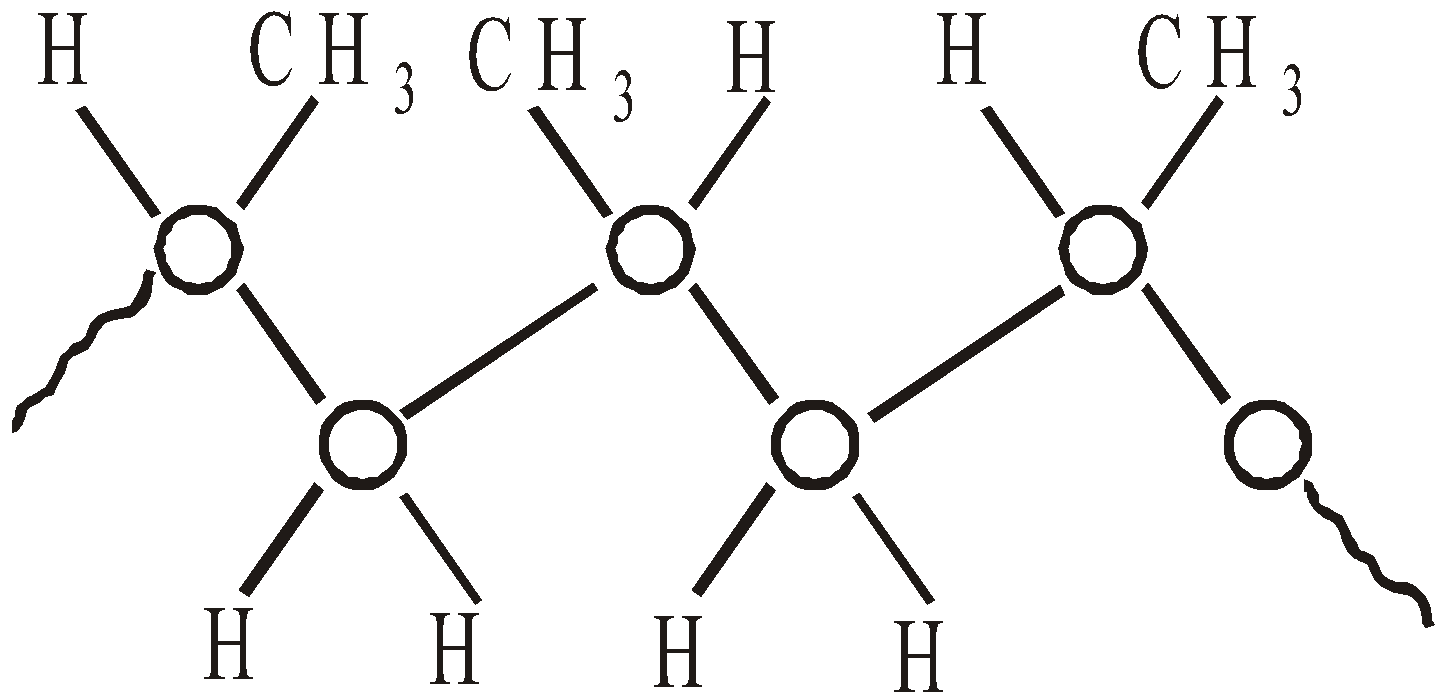
- Atactic – with random distribution of methyl groups
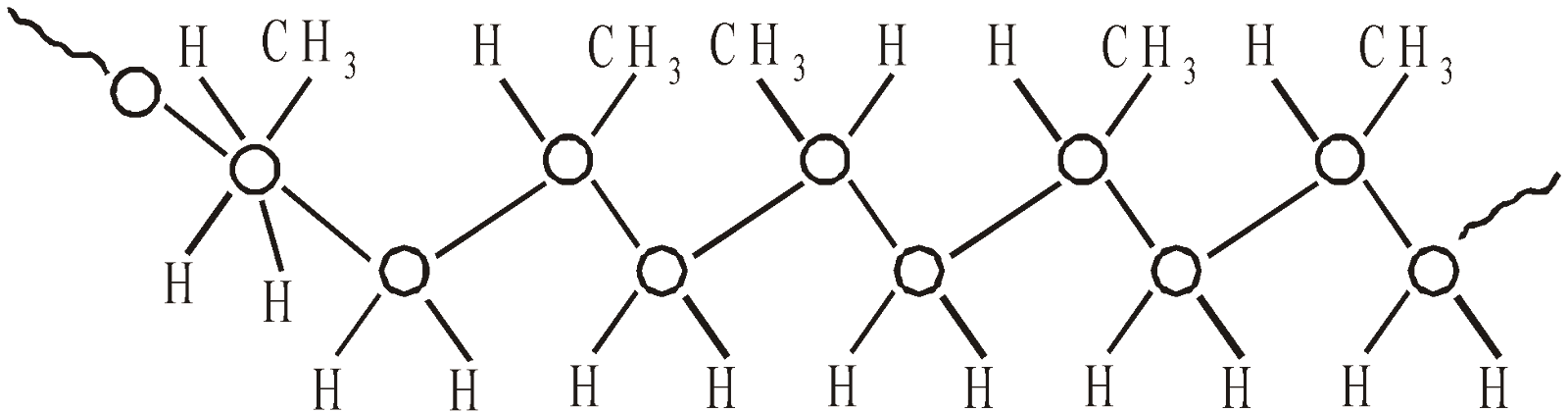
(Above polymers are obtained by coordination polymerisation)
CLASSIFICATION BASED UPON MODE OF SYNTHESIS
- Addition polymers – Polymers formed without the elimination of any elements and the molecular weight of the polymer is the exact multiple of the monomer unit are known as addition polymers
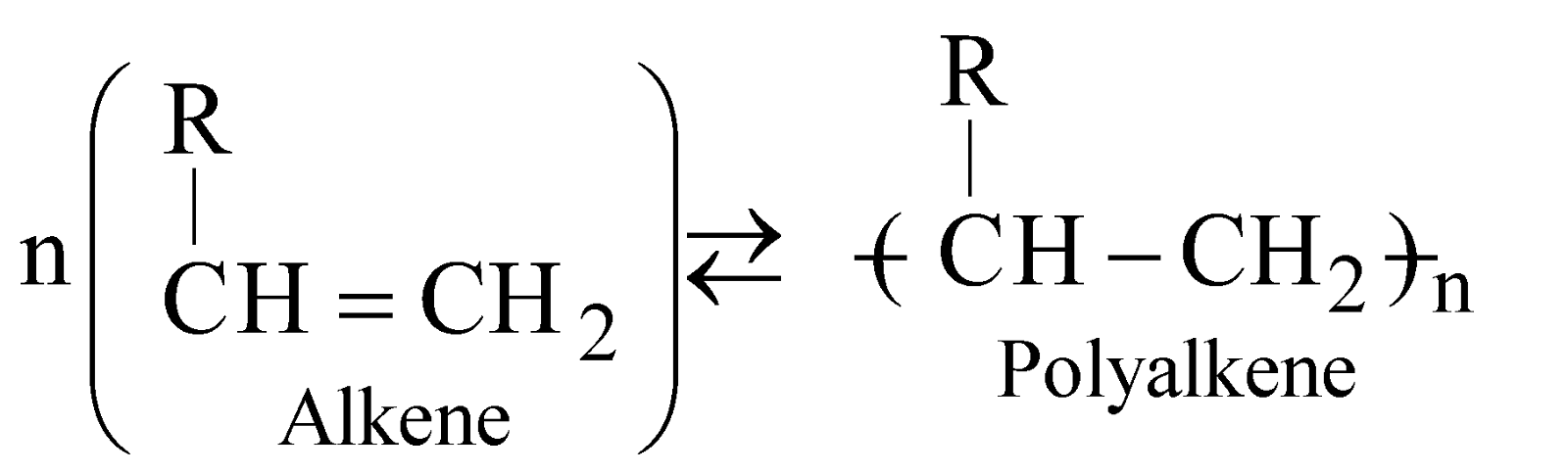
- Condensation polymers – They are formed with elimination of certain elements in the form of H2O, C2H5OH, NH3 etc. and molecular weight of polymer is not exact multiple of monomer unit eg. terephthalic acid condenses with ethylene glycol to give polyester
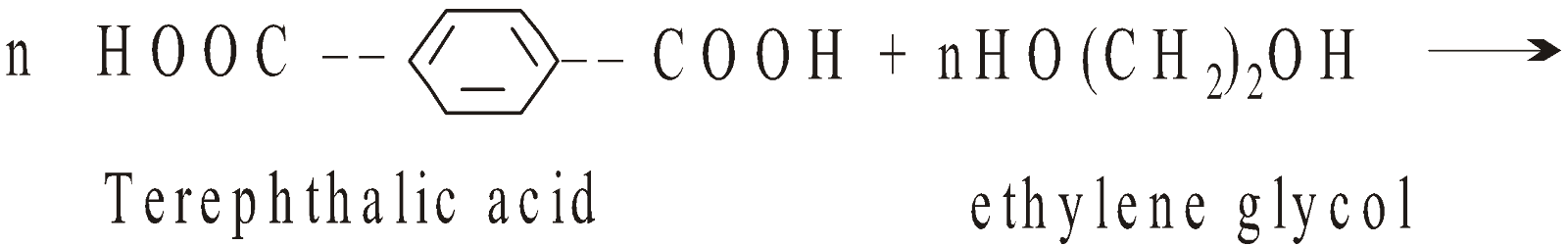
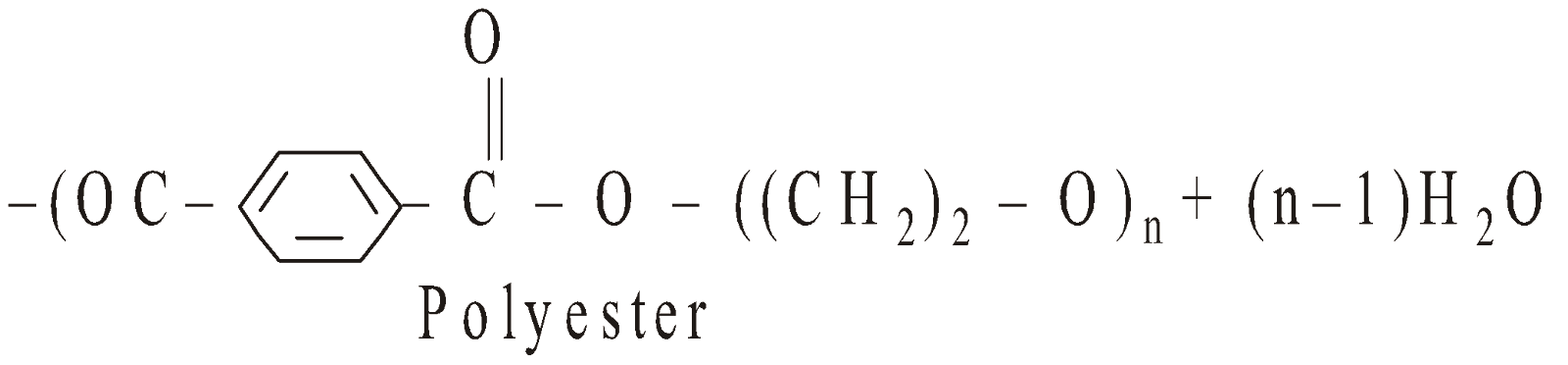
CHAIN GROWTH AND STEP GROWTH POLYMERS
- Chain growth polymers – In chain growth polymerisation each step consumes a reactive particle and produces another similar particle.
- Step growth polymers – Polymerisation proceeds through various steps independent of each other and it happens when monomer units contain more than one functional group. For example formation of nylon 66, bakelite etc proceed by step growth polymerisation.
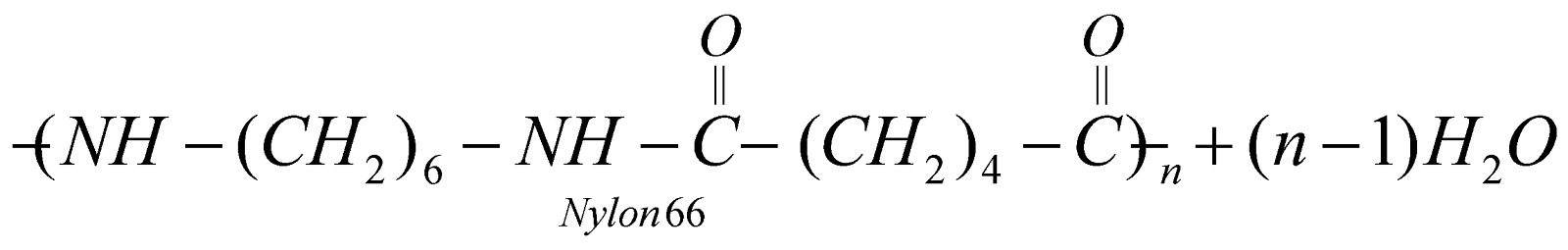
CLASSIFICATION ON THE BASIS OF PHYSICAL PROPERTIES AND INTERPARTICLE FORCES
- Elastomers – They can be easily stretched and can be made rigid to some extent by adding cross linking agents eg vulcanised rubber. They possess weakest intermolecular forces.
- Thermoplastics – They become soft on heating and harden on cooling. eg polythene, polystyrene. They can be worked up again and again. Forces between molecules are intermediate of elastomers and fibers.
- Thermosetting – Such polymers undergo chemical changes when heated and set to hard mass when cooled eg Bakelite. Such polymers can not be reworked. There is excessive cross linking on heating and three dimensional network of bonds.
- Fibres – Linear polymers containing polymeric units joined by hydrogen bonding. They can be woven into fabrics. Rayons, nylons are the examples.
FORMATION OF ADDITION POLYMERS
They can be obtained by
- Free radical polymerisation
- Cationic polymerisation
- Anionic polymerisation
- Ziegler-Natta polymerisation or coordination polymerisation
VULCANIZATION OF RUBBER
- Heating of rubber with sulphur which causes formation of sulphur bridges between molecules which are then cross linked is known as vulcanization of rubber.
- The vulcanised rubber is more elastic than natural rubber.
- Vulcanization may be brought about by free radical generators (peroxides, azo compounds) and metal oxides (ZnO or MgO) also.
ANTIOXIDANTS
Natural rubber is very sensitive to oxidation by air and ozone which can be inhibited by adding antioxidants. They undergo easy oxidation and prevent the oxidative degradation of rubber. eg.
N-phenyl- naphthylamine and Di-
naphthylamine and Di-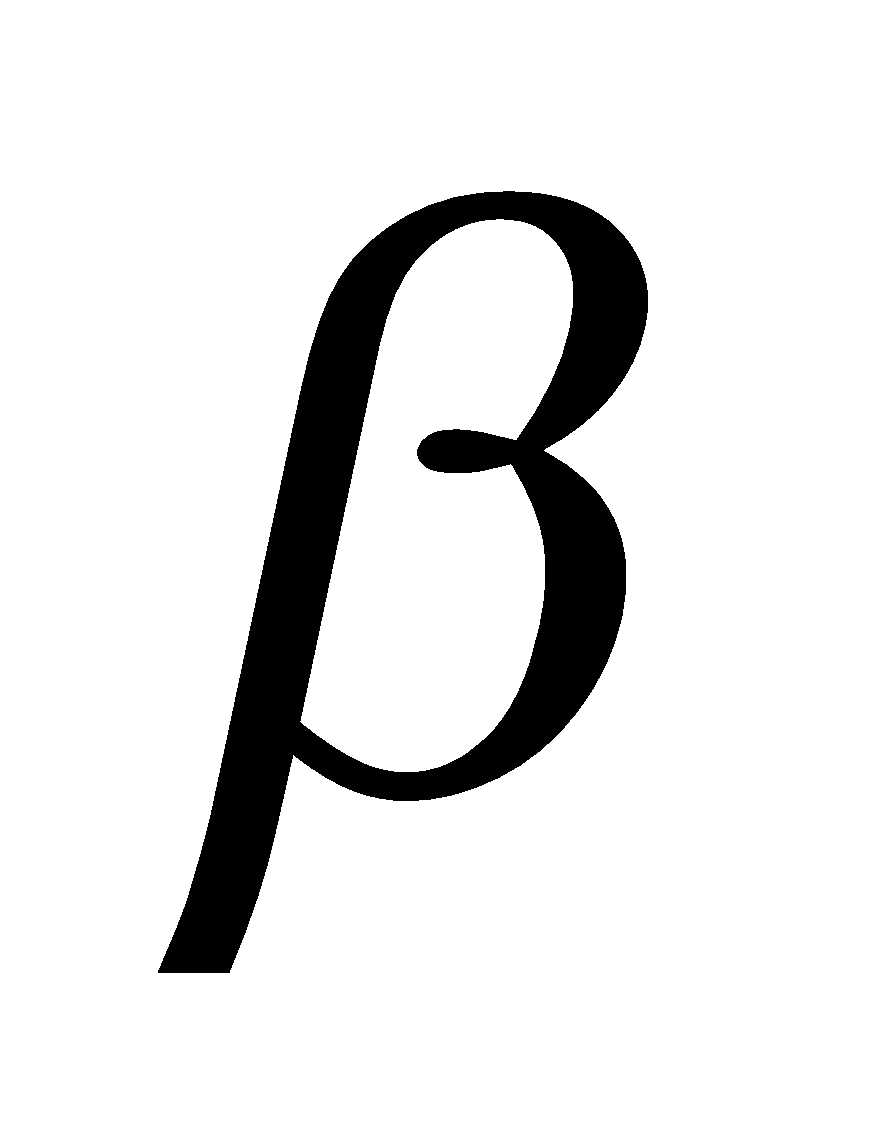 -naphthyl-p-phenylenediamine.
-naphthyl-p-phenylenediamine.
N-phenyl-
MOLECULAR MASS OF POLYMERS
It is of two types
- Number average molecular mass (Mn–) is given by
Where N1 number of molecules having molecular mass M1 and so on. It is measured by osmotic pressure measurement.
- Mass-average molecular mass
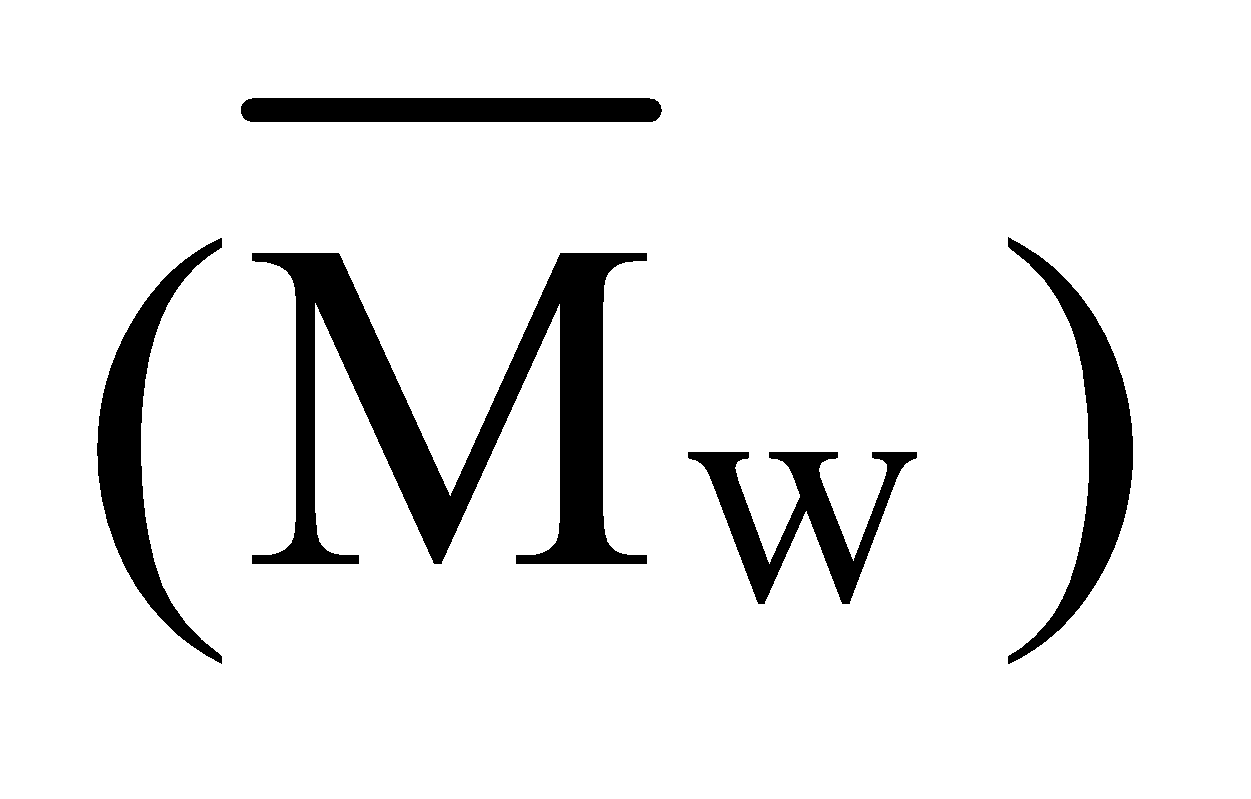 is given by
is given by
It is determined by ultracentrifugation or sedimentation.
POLYDISPERSITY INDEX
It is the ratio of mass average molecular mass to the number average molecular mass
PDI = 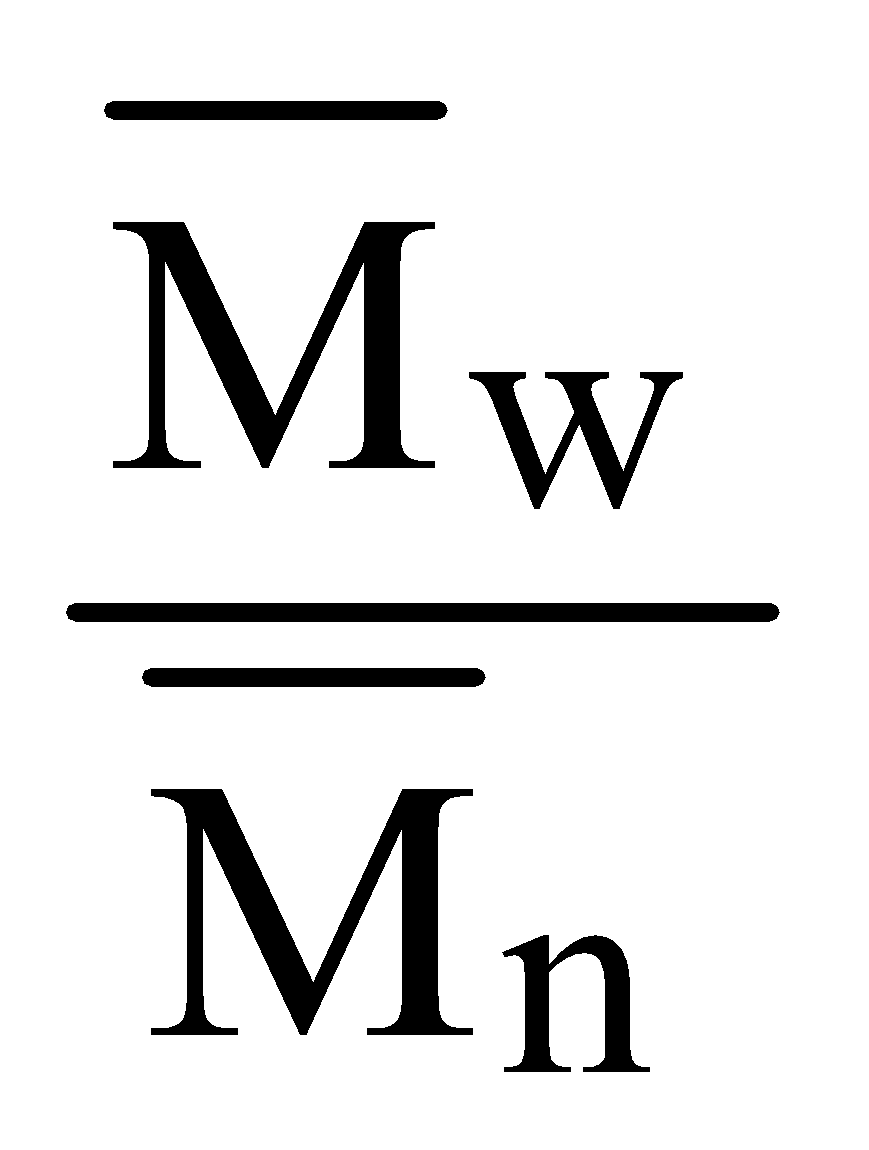
When PDI = 1 or nearly 1, we have monodisperse system in which all molecules have identical molecular mass. Natural polymers are monodisperse and synthetic polymers are polydisperse i.e. PDI > 1 for such polymers.
IMPORTANT ADDITION POLYMERS
POLYETHYLENE
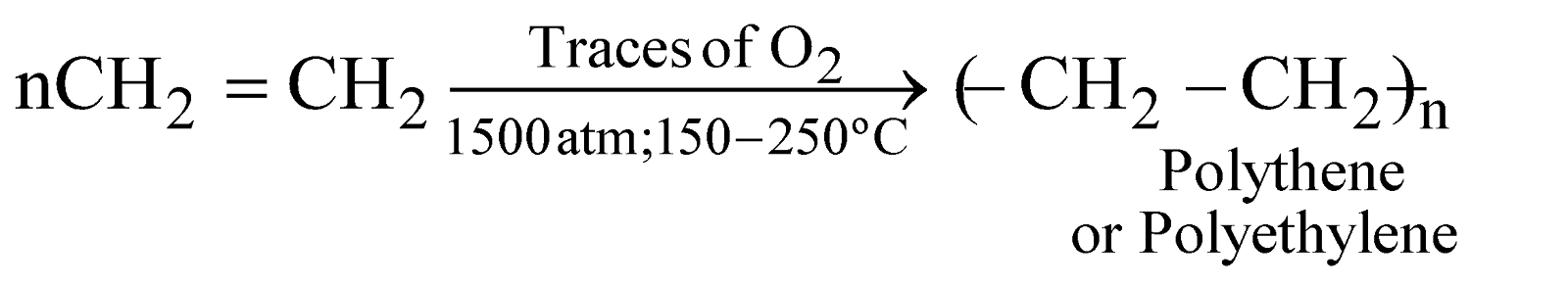
Free radical initiator gives low density polythene and ionic catalyst gives high density polythene. It is white, translucent, rigid, linear, used for making tubes, pipes, coated wires, insulator parts. etc.
POLYPROPYLENE
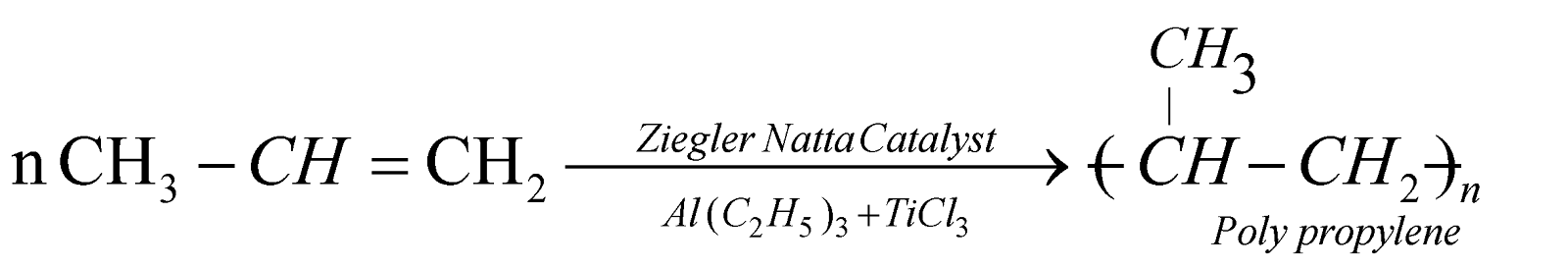
It has good hardness, stiffness, resistance. Used for making ropes, carpets, washing machine parts etc.
POLYSTYRENE
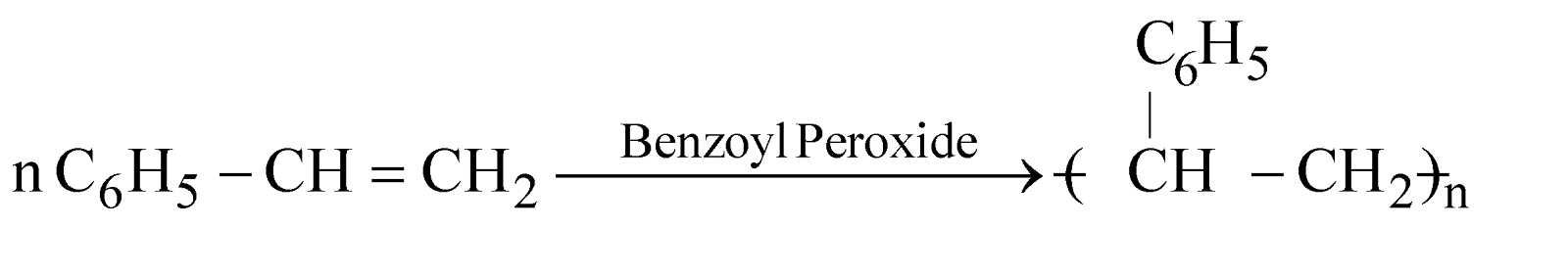
It is transparent, light, moisture resistant used for battery cases, refrigerator parts, electric insulators, combs, buttons TV cabinets. Its trade name is Styrofoam or styron.
POLYMETHYLMETHACRYLATE, LUCITE OR PLEXIGLASS
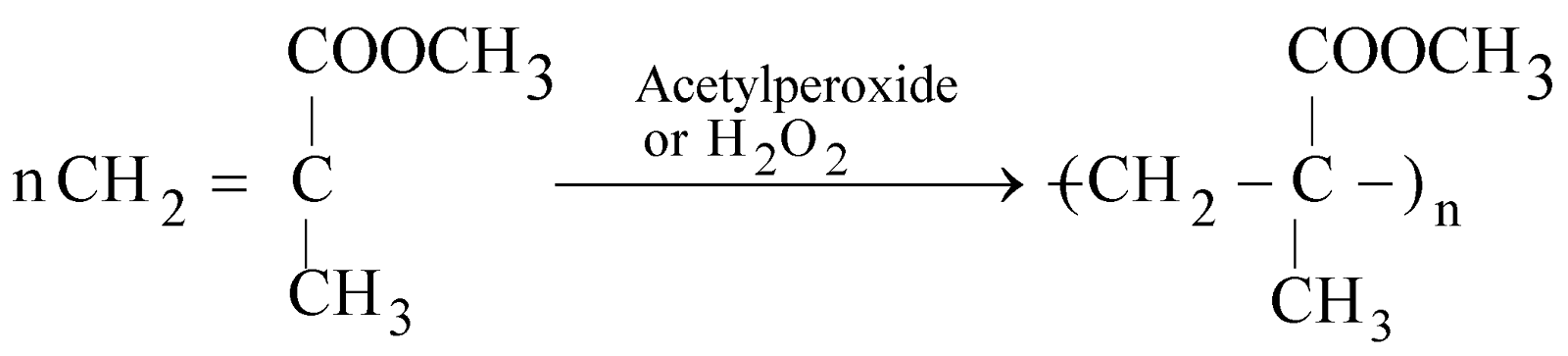
Methyl Methacrylate (MMA) PMMA
It is hard, fairly rigid. It is used for making lenses, artificial eyes, dentures, aircraft windows.
POLYVINYL CHLORIDE (PVC)
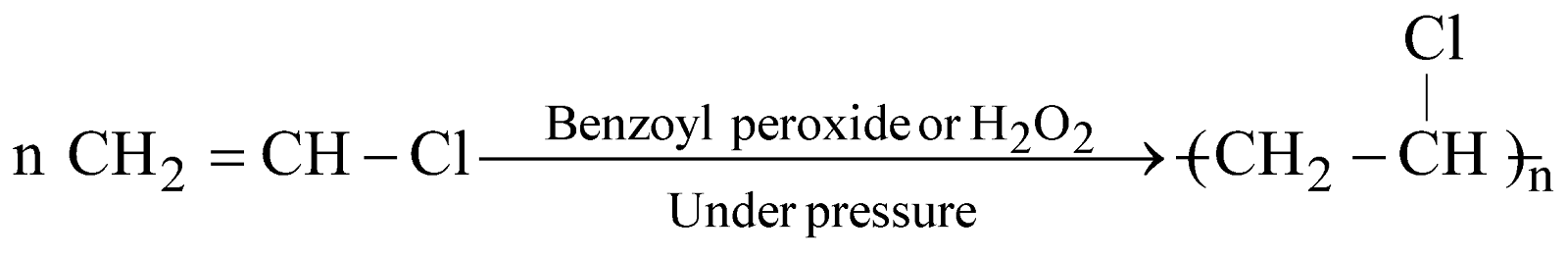
It is resistant to light, atmospheric oxygen, chemically inert. It is used for electrical insulators, floor covering, safety helmets etc.
TEFLON, POLYTETRAFLUOROETHYLENE (PTFE) OF FLUON
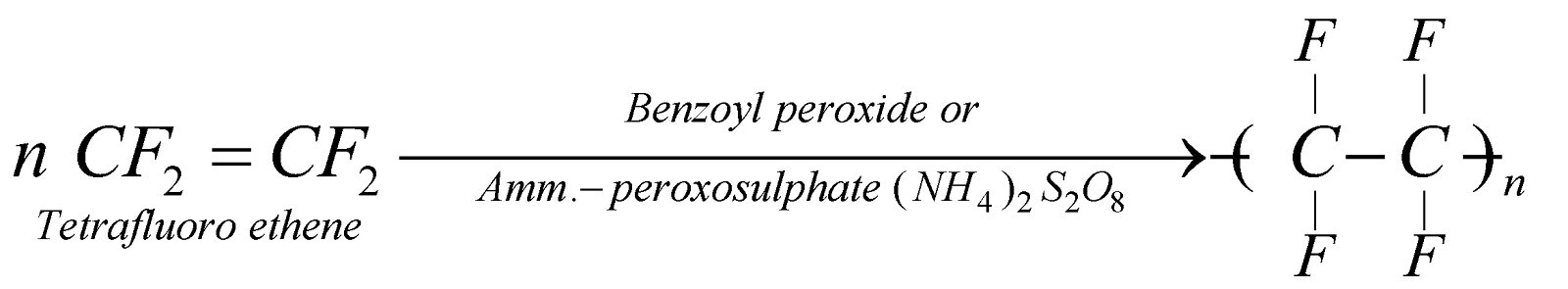
It is extremely tough, resistant to heat and chemicals. It is used for making gaskets, pump parts, coating utensils, high frequency insulator
POLYACRYLONITRILE (PAN), ACRILON OR ORLON
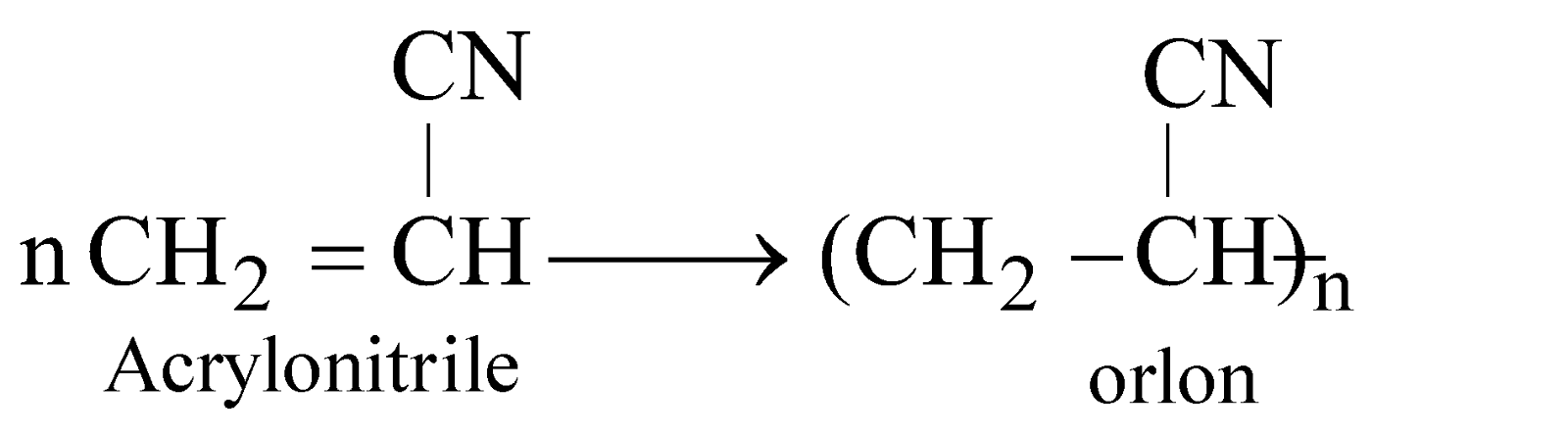
It is hard used in preparing cloths, carpets
RUBBERS
BUNA – S OR SBR OR GSR (GOVERNMENT STYRENE RUBBER)
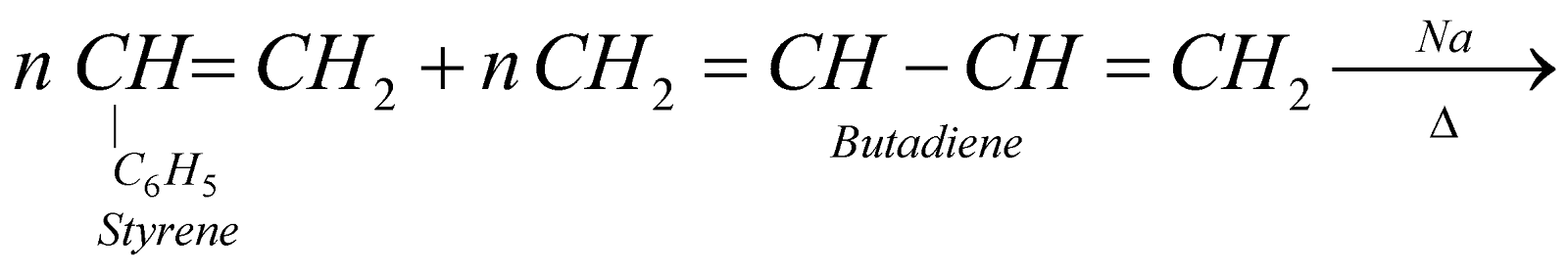
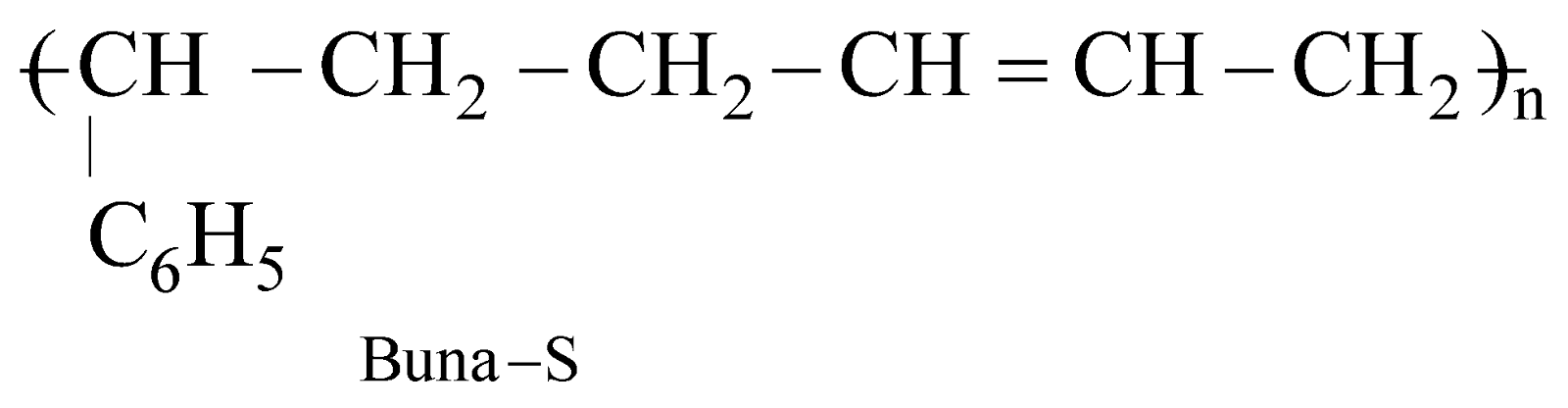
It is used for manufacture of tyres, floor tiles, gaskets, cable insulators etc.
NITRILE RUBBER (GR-A OR BUNA – N)
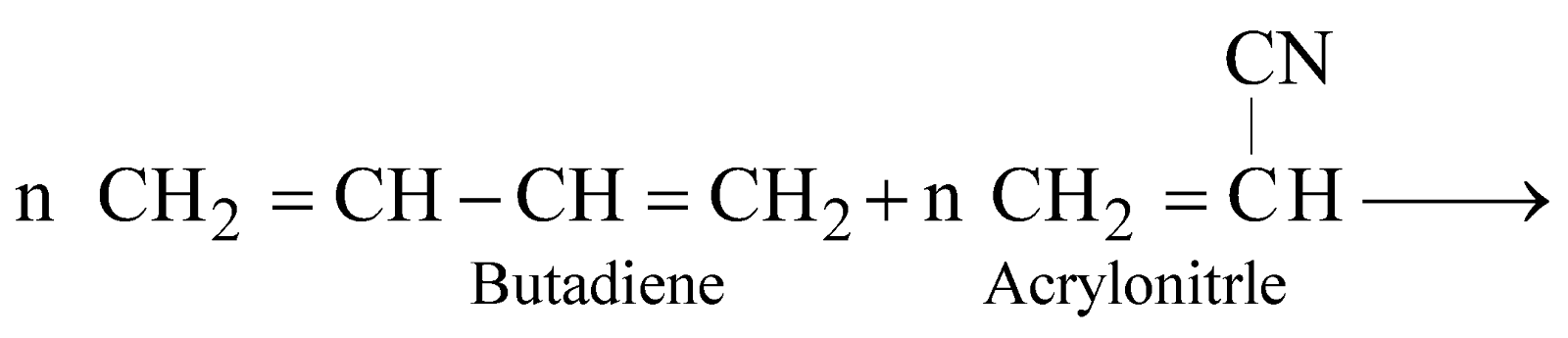
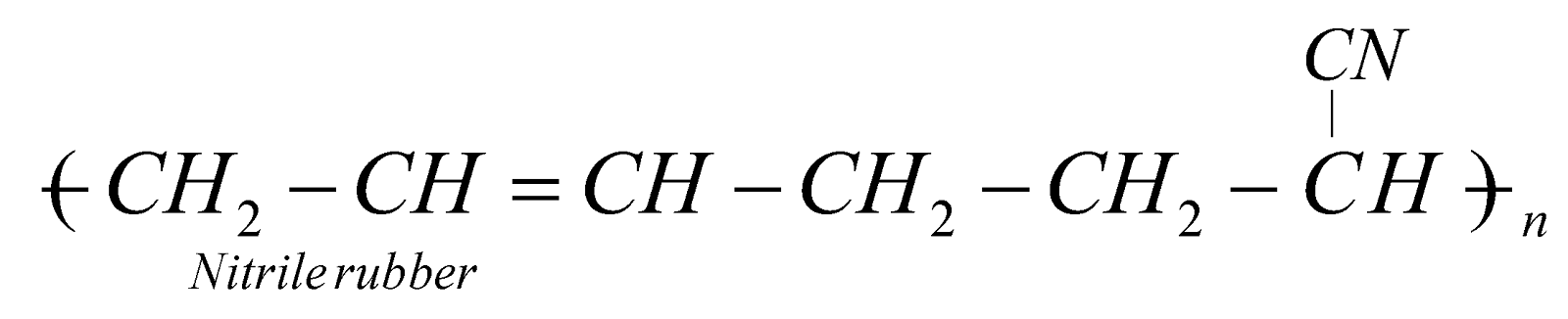
Excellent resistant to heat and chemicals. It is used for making conveyor belts, printing rollers, automobile parts.
NEOPRENE
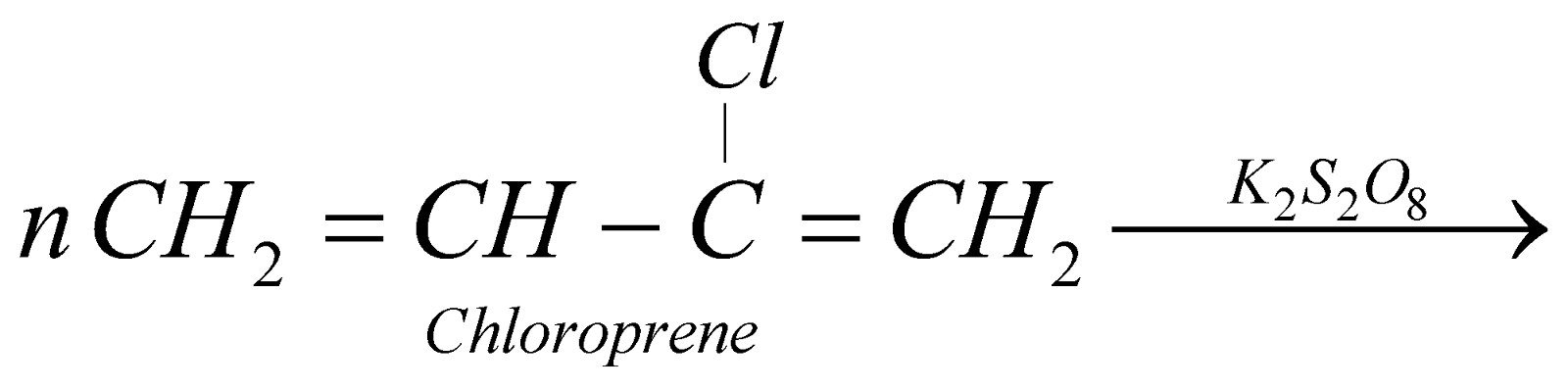
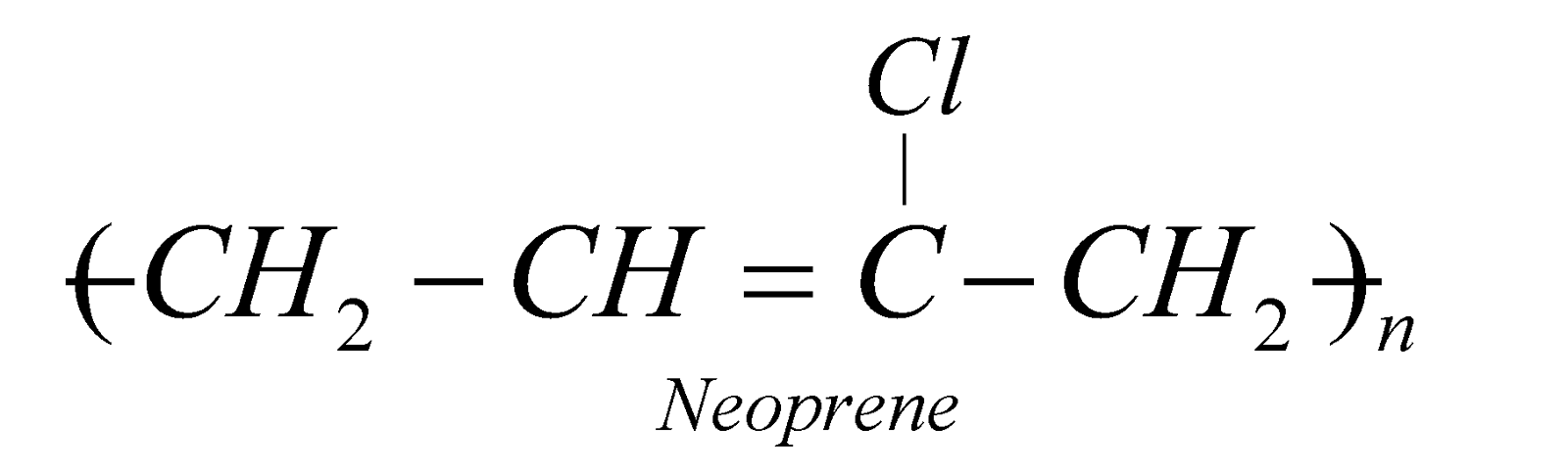
It is resistant to heat and chemicals. It is used for making protective clothing, reaction vessels, floor tiles etc.
HEAVY RUBBER
It is cis polyisoprene having length of repeat unit 8.1 Aº.
GUTTA PERCHA
It is trans polyisoprene having length of repeat unit 4.72Aº.
IMPORTANT CONDENSATION POLYMERS
NYLONS
Synthetic polyamides are known as Nylons
NYLON 66
Copolymer of adipic acid (6C) and hexamethylenediamine (6C)
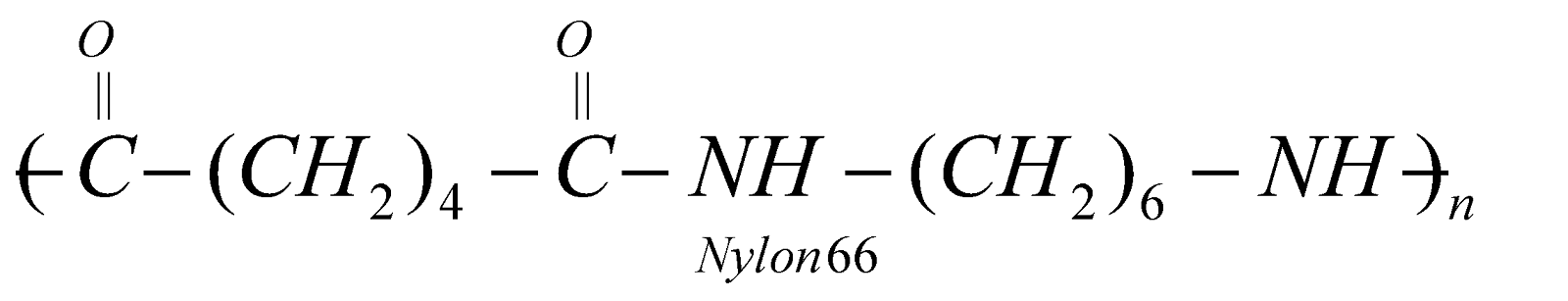
It has high tenacity and elasticity. It is resistant to abrasion and not affected by sea water. It is used for reinforcement of rubber tyres, manufacture of parachute, safety belts, carpets and fabrics.
NYLON 6
Homopolymer of Caprolactam (6C)
Homopolymer of Caprolactam (6C)
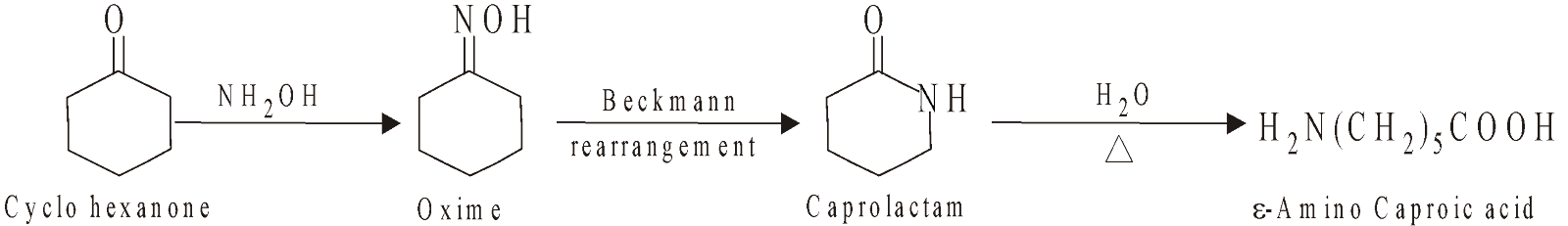
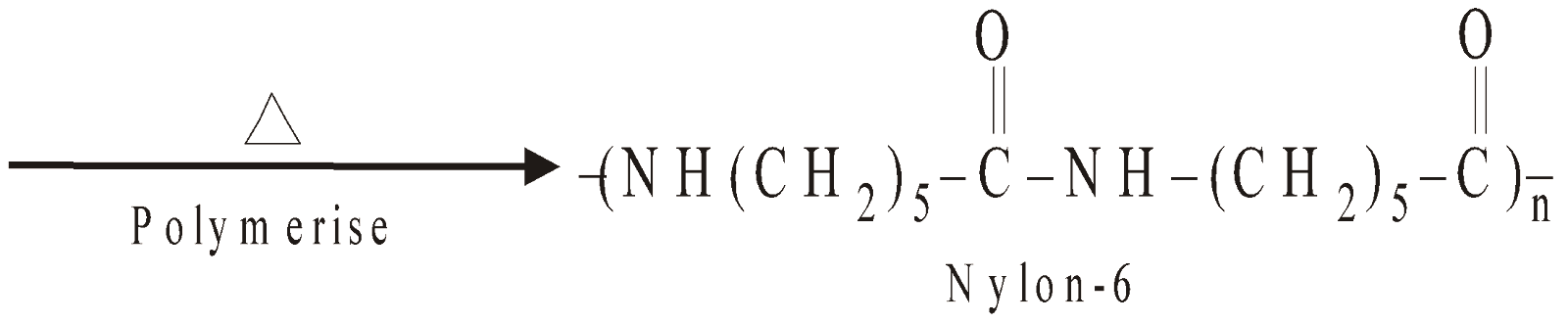
NYLON 6,10
Copolymer of hexamethylene diamine (6C) and sebacoyl chloride (10C)
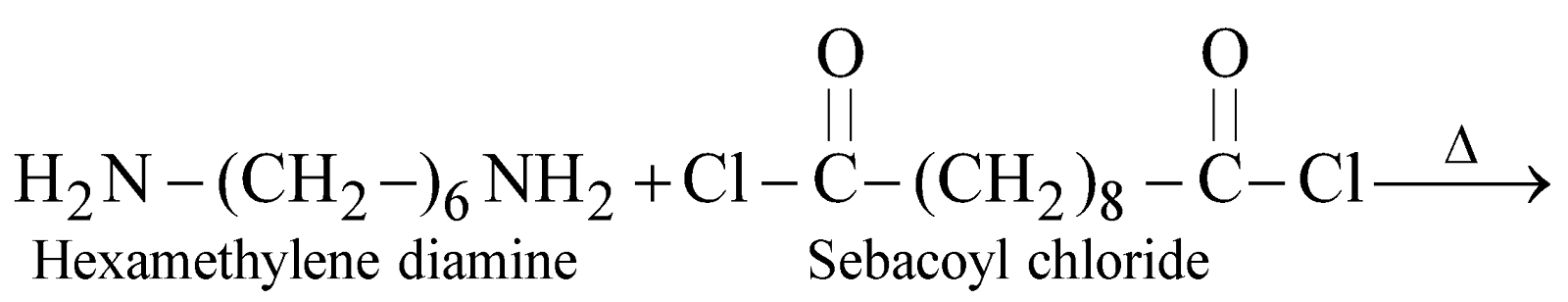
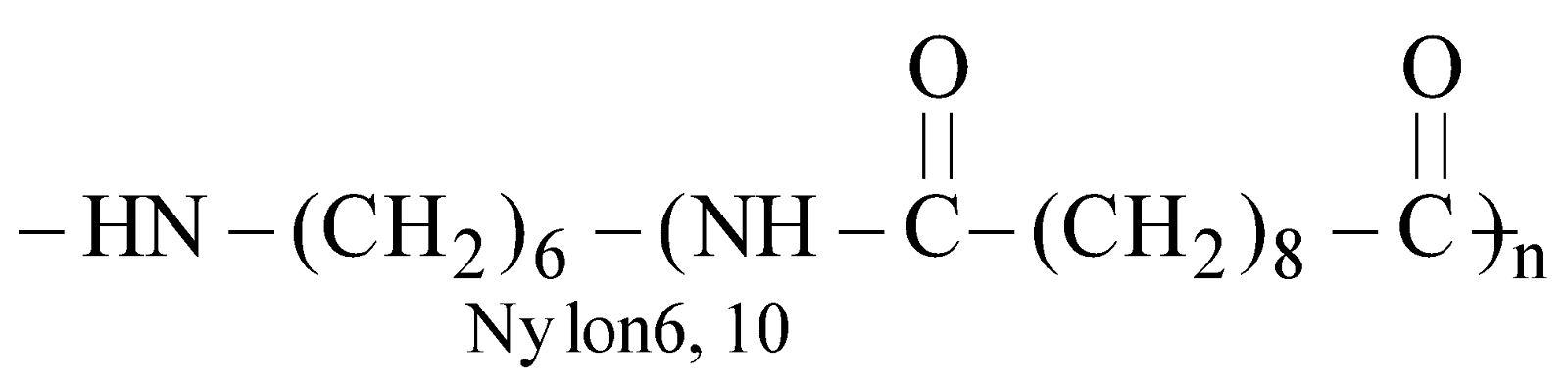
It is used for making brush bristles
KEVLAR
It is aromatic polyamide resembling nylons.
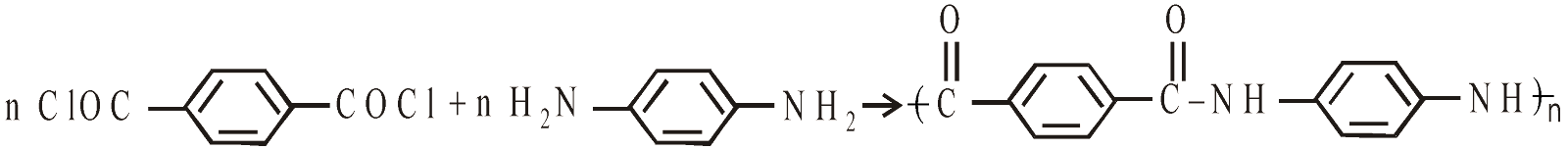
Terephthalic acid p-phenylenediamine dichloride Kevlar
It is used in aircraft industries, bullet proof vests, helmets, ropes cables.
POLYESTERS
Condensation polymers of a dibasic acid and a diol
TERYLENE (DACRON) / POLYETHYLENE TEREPHTHALATE (PET)
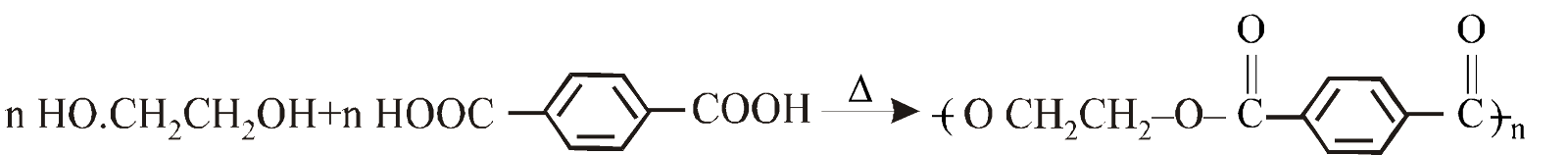
Ethylene glycol Terephthalic acid Terylene
It is resistant to mineral and organic acids. It is used for blending with wool to provide better crease, in safety helmets and aircraft battery boxes.
GLYPTAL OR ALKYD RESIN
Condensation polymers of dibasic acids and polyhydroxy alcohols
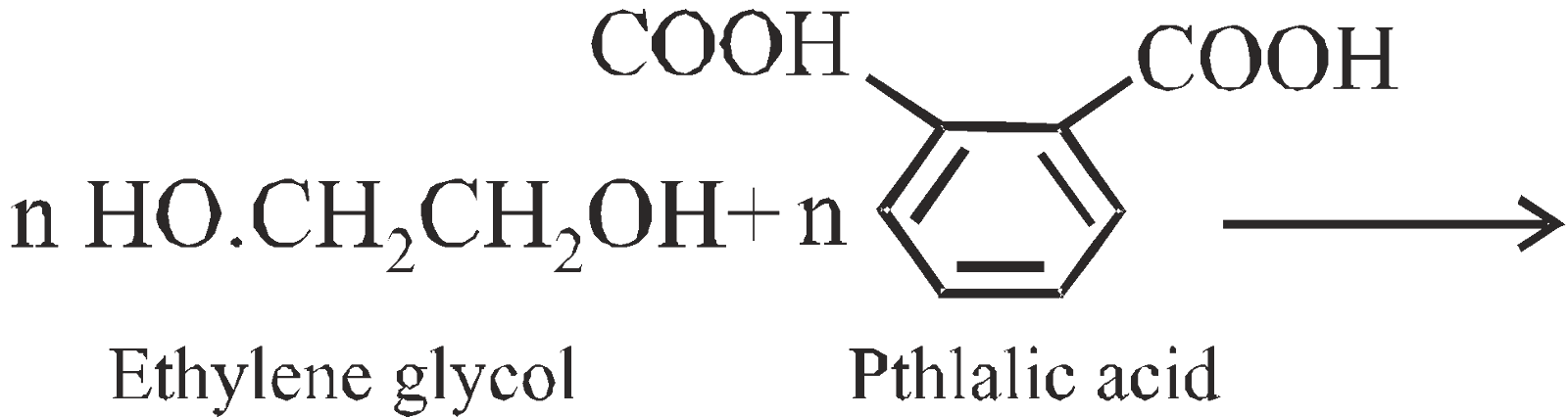
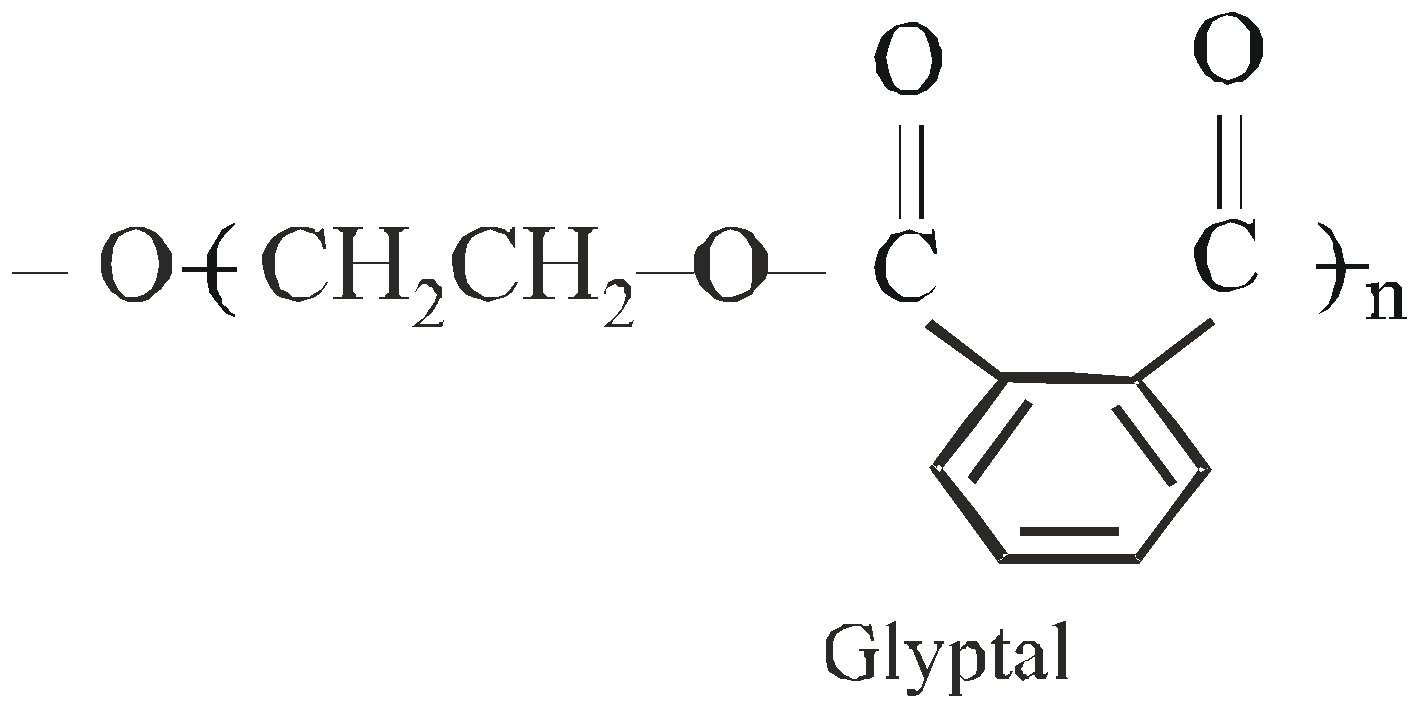
It is a cross linked copolymer. It is used for making good insulators, sheets, rods, switches, lacquers and adherent paints.
THERMOSETTING RESINS
BAKELITE
Phenol formaldehyde resin

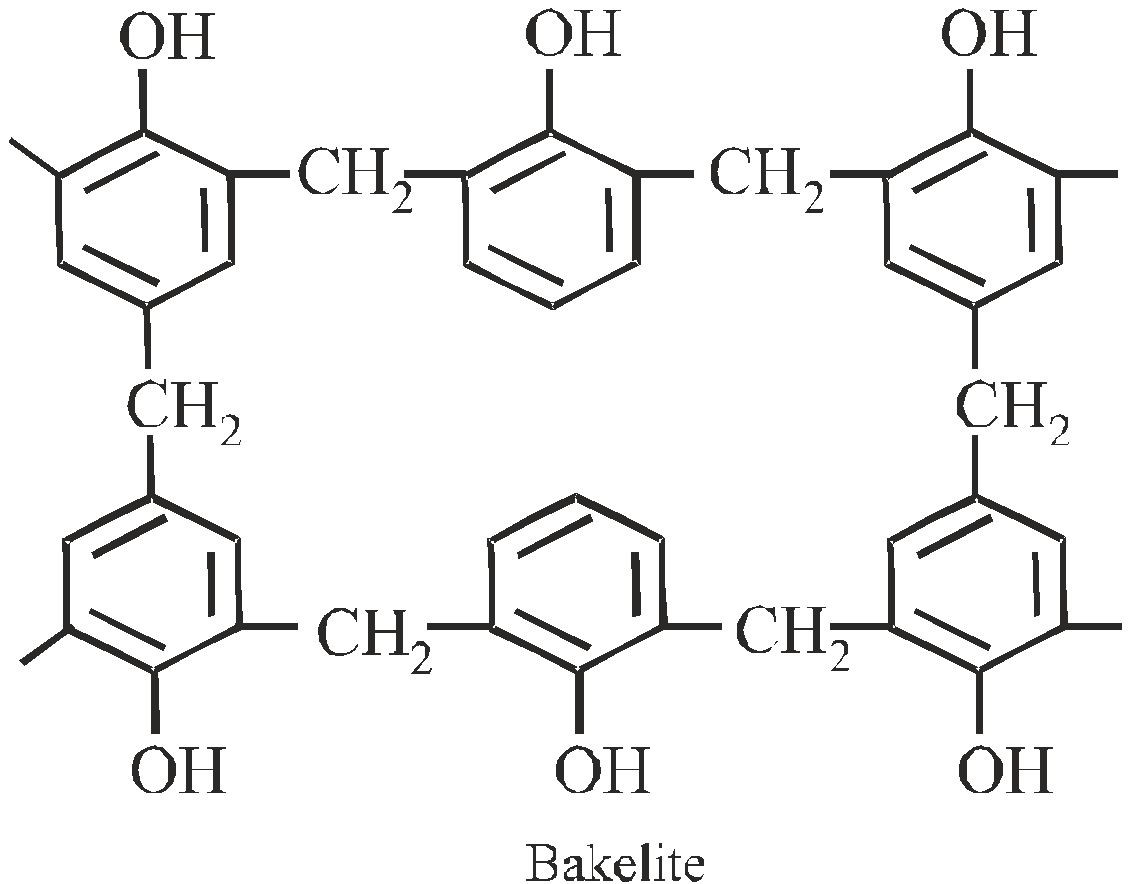
High degree polymerisation leads to rigid, hard, scratch resistant, infusible, attacked by alkalies phenolic resins. They are used for making switches, plugs telephone parts. Sulphonated bakelites are used as ion – exchange resins in water softening.
Low degree polymerisation gives bonding glue, preparation of varnishes and lacquers.
UREA FORMALDEHYDE RESIN
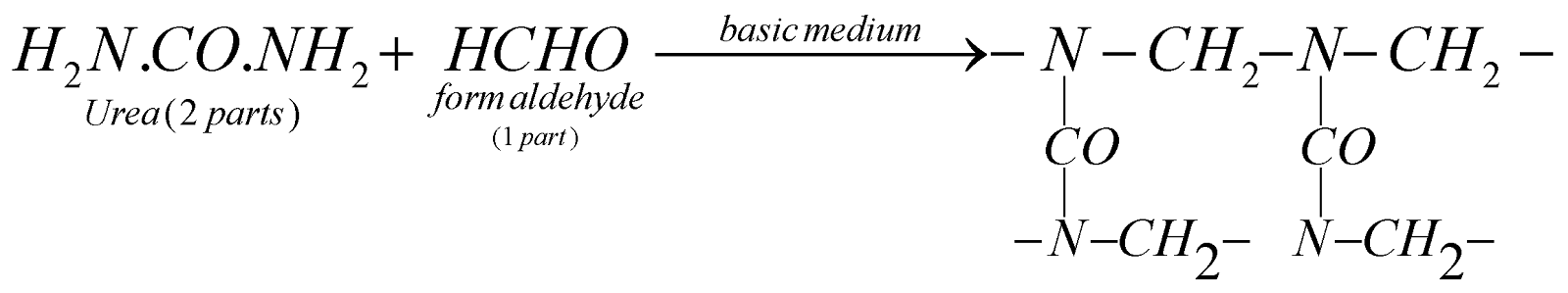
Urea formaldehde resin
(Crosslinked polymer)
It is used for bonding grinding wheels, insulation, cation exchanger and decorative articles.
MELAMINE FORMALDEHYDE RESINS (MF RESIN)
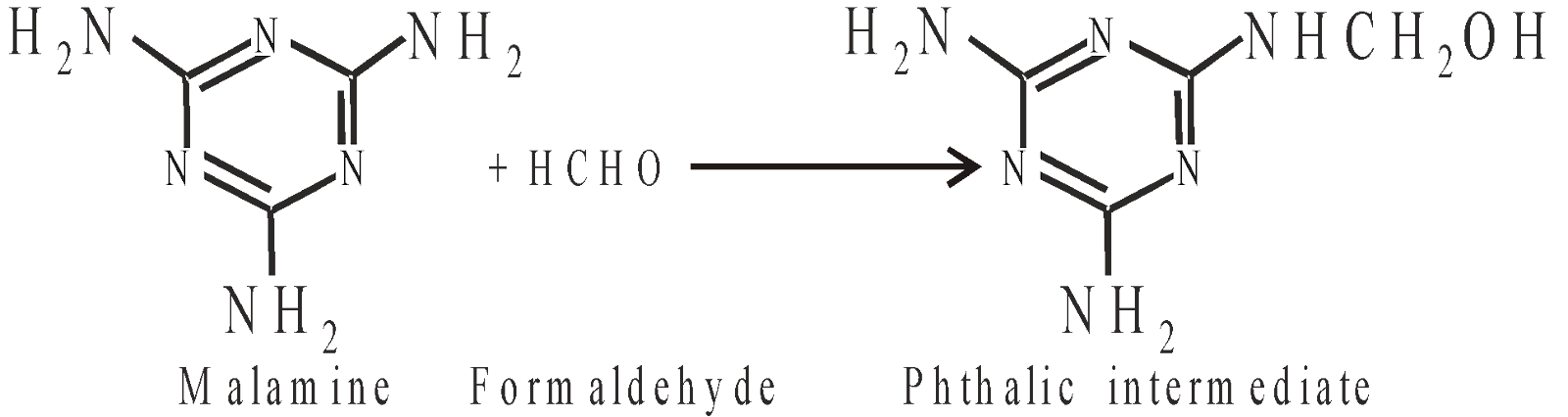
It is better than UF resin and used in making decorative laminates crockery under the trade name melmac, plywood industry as adhesive.
POLYURESTHANES
Polymers of diisocyanate and diol eg. : Perlon-U

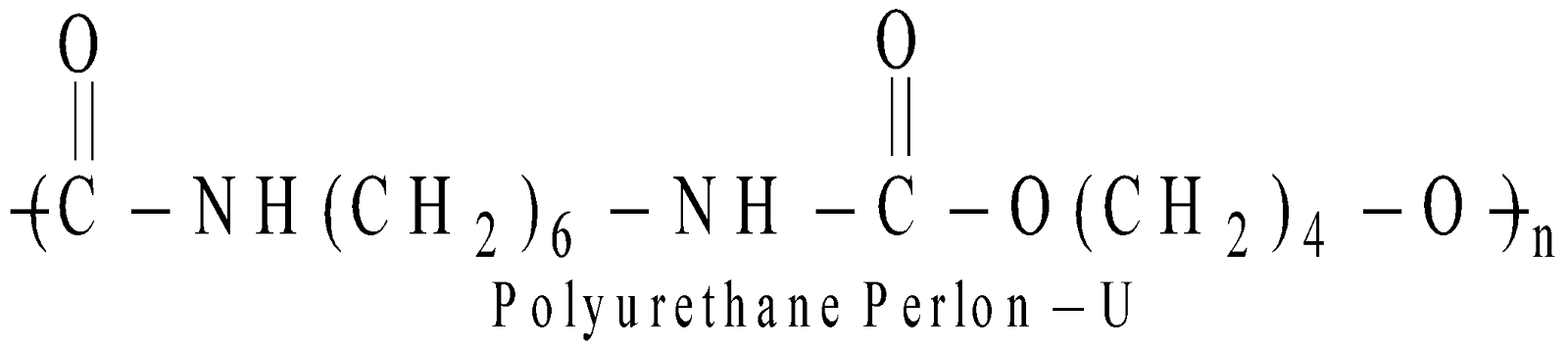
It is substitute of leather. They are used as foams, films adhesives, gaskets etc.
SILICON RESINS
Different organo-silicon chlorides can be polymerised by carefully controlled hydrolysis giving silicon resins. They contain alternate silicon oxygen structure.
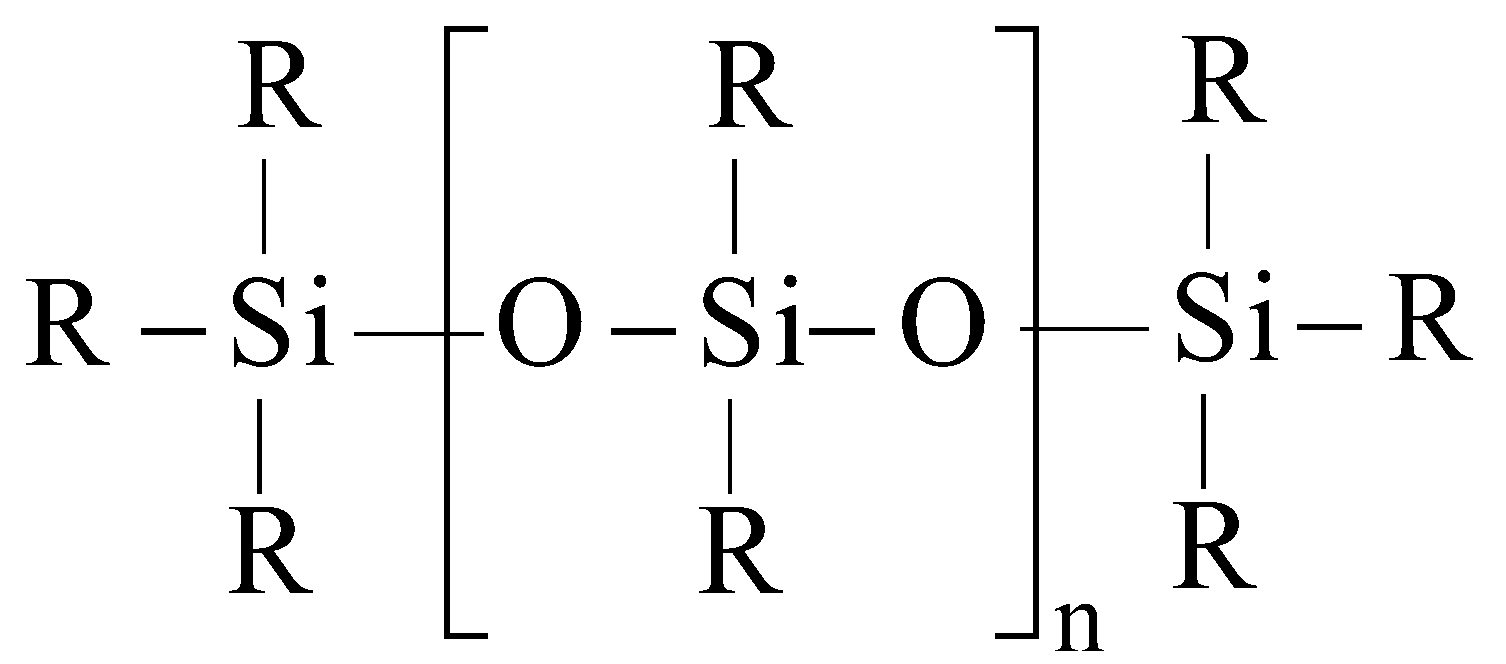
where R = alkyl or phenyl radical
Silicones may be
- liquid silicones or silicone oils
- silicon greases
- silicon rubbers
- solid silicones
RESIN
Resin is a low molecular weight polymer used as binder, fusible and mouldable. It changes into infusible cross-linked form during moulding in presence of catalyst.
RAYON
It is artificial silk. Actually the term includes all synthetic fibres manufactured from cellulose. The manufacturing can be carried out by
- Acetate process
- Viscose process
PLASTICIZERS
The substances added to resins to increase their plasticity and flexibility are known as plasticizers. Examples are, vegetable oils (non drying), phosphates (tributyl phosphate, triphenyl phosphate etc), esters of oleic, stearic or phthalic acids and camphor etc.
Reclaimed rubber: Rubber obtained from waste rubber articles.
Reinforced rubber or plastic : They are obtained by adding solid fillers. They are also known as filled plastics or rubbers.
