Topic 2 : Molecular Biology
2.1 Molecules to metabolism
Molecular biology is the chemistry of living organisms
- “DNA makes RNA makes protein”.
- The information in this flow cannot be reversed and the protein generated cannot change the RNA or DNA
- There are many molecules important to living organisms including water, carbohydrates, lipids, proteins and nucleic acids
- Proteins are one of the most varied macromolecules, performing many cellular functions, including catalyzing metabolic reactions (enzymes)
Carbon-based life
- Covalent bonds are the strongest type of bond between atoms. Stable molecules can be formed
- Carbon atoms contain four electrons in their outer shell allowing them to form four covalent bonds with potential four other different atoms.
- Carbon has a few unique bonding properties – the most important of which is its ability to form long chains of carbon. No other element can bond like carbon does.
- Since carbon-carbon bonds are strong and stable, carbon can form an almost infinite number of compounds
- Covalent Bonds are chemical bonds formed by the sharing of a pair of electrons between atoms. The nuclei of two different atoms are attracting the same electrons.
- Carbon compounds including carbohydrates, lipids, proteins and nucleic acids
Carbohydrates
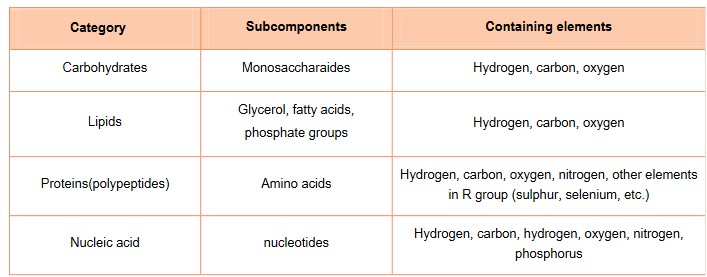
- Contain carbon, hydrogen and oxygen
- Organic compounds consisting of one or more simple sugars
- Monomers follow the general basic formula of \((CH_2O)_n\)
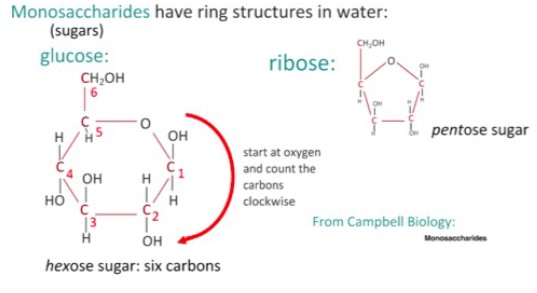
- Monomers are commonly ring shaped molecules
- Many carbohydrates are used for energy or structural purposes
- Carbohydrates contain starch, glycogen and cellulose
Lipids
- Lipids are a group of organic molecules that are insoluble in water but soluble in non-polar organic solvents
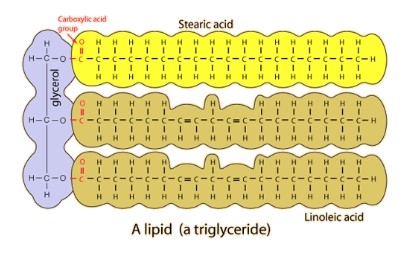
- Common lipids include triglycerides (fats – solid at room temperature and oils – liquid at room temperature), phospholipids and steroids
- Some lipids function in long-term energy storage. Animal fat is a lipid that has six times more energy per gram than carbohydrates.
- Some examples of lipids are triglycerides, steroids, waxes and phospholipids
- Animal fats (saturated) are solid at room temperature and plant fats (unsaturated) are liquid at room temperature
- Is made by glycerol + fatty acid
Protein
- Contain carbon, hydrogen, oxygen and nitrogen
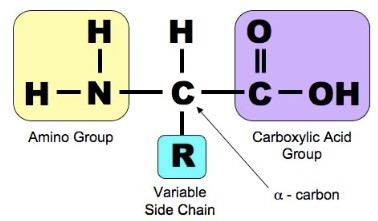
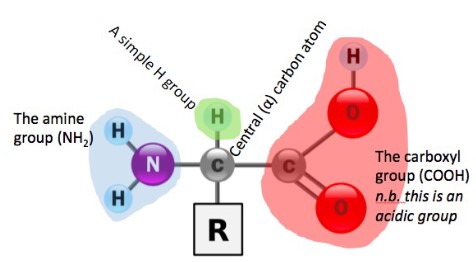
- Proteins are large organic compounds made of amino acids arranged into one or more linear chains
- Proteins are distinguished by their “R” groups. Some of these also contain sulphur
Nucleic acid
- Contain carbon, hydrogen, oxygen, nitrogen and phosphorus
- Chains of sub-units called nucleotides
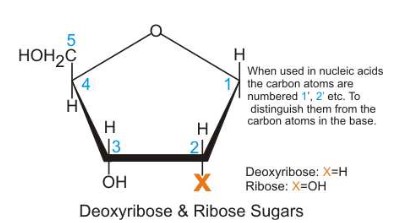
- Nucleotides consist of base, sugar and phosphate groups covalently bonded together
- The bases of DNA are Adenine, Thymine, Cytosine, and Guanine; In RNA, Uracil substitutes for Thymine
- If the sugar is ribose then the nucleic acid formed is RNA if the sugar is deoxyribose then DNA is formed
Identification of biochemical
- The generalized formula for carbohydrates is \(CH_2O\). All carbohydrate contain C, H and O
- Proteins also contain C,H, O but they all have N. Some proteins also contain S in their R-groups
- Lipids contain C, H and O as well, but in different ratios and much less O then carbohydrates
• Amino acids
• Fatty acid
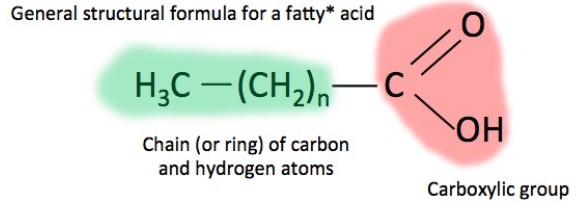
• Glucose
Metabolism
- Metabolism is the set of life-sustaining chemical reactions within the cells of living organisms.
- These reactions are catalyzed by enzymes and allow organisms to grow and reproduce, maintain their structures, and respond to their environments.
- Many of these reactions occur in the cytoplasm, but some are extracellular including digestion and the transport of substances into and between different cells
- The word metabolism can refer to the sum of all chemical reactions that occur in living organisms
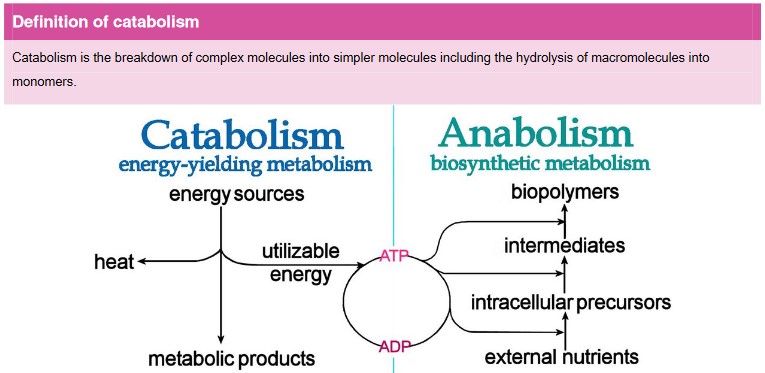
Anabolism & catabolism
• Anabolic reactions require energy as you are building large molecules from small ones (takes energy to build things)
• Some anabolic processes are protein synthesis, DNA synthesis and replication, photosynthesis, and building complex carbohydrates, such as cellulose, starch and glycogen
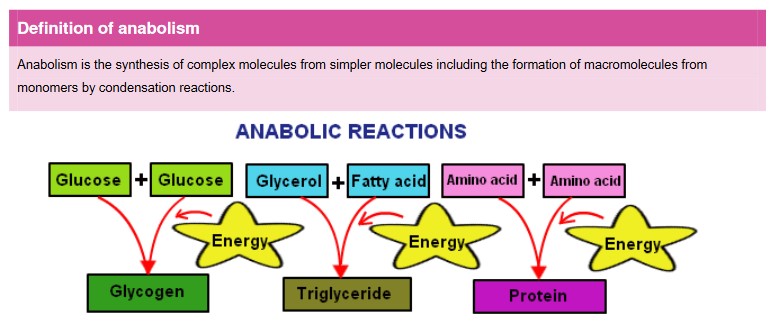
- Catabolism are reactions that break down larger molecules into smaller ones or their component parts
- Catabolic reactions release energy (sometimes captured in the form of ATP)
- Some examples of catabolic reactions are digestion of food, cellular respiration, and break down of carbon compounds by decomposers
- Condensation makes bond, release water, take in heat, as an anabolism reaction, which is endothermic
- Hydrolysis breaks bond, give out heat, as a catabolism reaction, which is exothermic
