ATOMIC PHYSICS
- The study of radieactive elements and their isotopes.
The protons and neutrons stick to ge the due to a binding force. Apart from the basic parts (protons, neutrons, electrons). they themselves are made up of tinier particles called quarks. There are 6 types of quarks:
- Up
- Down
- Top
- Bottom
- Chare
- stem
Radioactivity is the process of spontaneous disintegration of the nucleus of an atom with the emission of radioactive rays, including Alpha, Beta and Gamma.
How to show an element:
Atomic mass $\leftarrow A$
Alanic number $z \mathrm{Na} \rightarrow$ symbol Number
- An element is only radioactive if it atomic mass is greater than 82 amu.
- Three main types of radioactive rays, Alpha, $a(\alpha)$, Beta $(\beta)$ and Gamma (y).
- Alpha particles are fast-moving helium nude i $\left(4_2^4 \alpha\right)$. They have no electrons and are positive.
- Beta particles are fast-moving electrons $(-j \mathrm{e})$, and are negative.
- Gamma rays have no charge, and are photons, or energy particles.
- Characteristics of Radioactive Rays:
- Ionization power is the number of charge (t) a ray con provide to the medium it flows in.
- Alp ha: Power of Ionization – (2). This is because it is 2 electrons short from a full shell.
- Beta: Power of Ionization – (1) They gain one electron from the medium travelled in
- Gamma: Power of Ionization -(70) Despite Gamma rays having no charge, they ionize the medium to a very low level due to their high speeds.
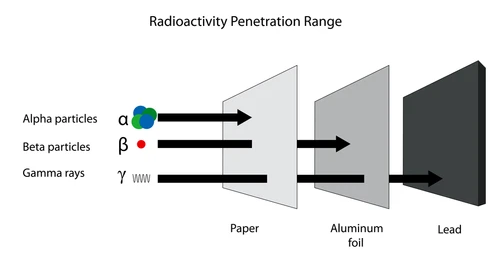
- Penetration Power (w hat different rays can pass through and what not:
- Alpha: Low (stopped by paper)
- Beta: Medium (stopped by Aluminum)
- Gamma: High (Stopped by lead/ concrete)
- Speeds
- Gamma- speed of light $\left(3 \times 10^8 \mathrm{~m} / \mathrm{s}\right)$
- Beta -Approximately speed of light
- Alpha – $10 \%$ of speed of light
- There are several uses of these radioactive rays
- Alpha rays are used for detecting the amounts of nutrients in certain crops.
- Beta rays can be used for investigating patients’ bodies and tracing organs without surgery.
- Gamma rays can be used for sterilising equipment, killing cancer cells, detecting breakages and leakages in pipes etc.
- To determine whether a radioactive ray is Alpha, Beta or Gamma, a device called a Geiger counter is used.
- A radioactive isotope (an element with an unstable number of neutrons) an undergo Radioactive Decay, then become stable. There are 4 types of day:
- Alpha Decay.
$
{ }_2^A X \rightarrow{ }_{2-2}^{A-4} Y+{ }_2^4 \mathrm{He}
$
- Beta Decay (electron):
$
{ }_z^A X \rightarrow{ }_{z+1}^A Y+{ }_{-1}^0 e
$
- Beta Decay (positron):
$
{ }_2^A X \rightarrow{ }_{2-1}^A Y+{ }_1 e .
$
- Gamma Decay:
Protons get closer to neutrons, Gamma ray is emmitted boo.
- Carbon Dating can be done on this principle (finding the age of a substance based on its emissions).
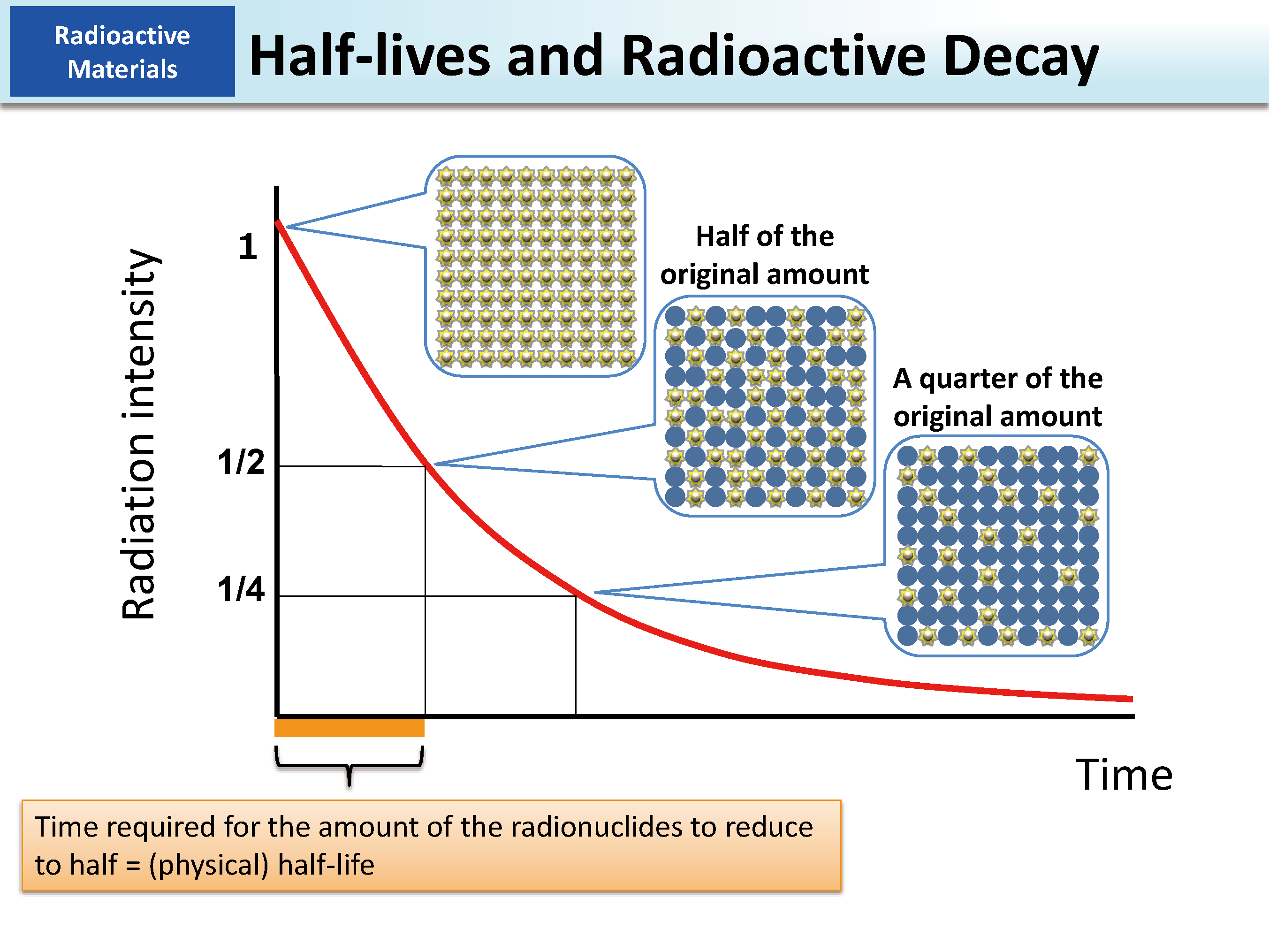
- Half-life (during which the number of atoms in a substance reduces to half). The time taken for number ofatons to get to $100 \%-50 \%, 50 \%-25 \%$ $25 \%$ to $12.5 \%$ and so on is the same
- A nuclear plant works on the principle of Nuclear Fission (the process of a neutron being bo barded onto a high. energy nucleus that splits).
- Two types of Nuclear Reactions:
- Controlled (Nuclear Reactor)
- Uncontrolled (Atomic Bomb)
- Example of a Nu clear Reaction.
$
\begin{aligned}
& { }_{92}^{235} \mathrm{U}+{ }_0^1 n \rightarrow{ }^{144} \mathrm{Ba}+{ }_{36}^{90} \mathrm{Kr}+3 \mathrm{O}_0^1 \mathrm{n} \\
& \text { energy } \\
&
\end{aligned}
$
- These 3 neutrons further carry out fission themselves.
- To convert between mass and energy:
$
E=m c^2, \text { speed of light }
$
speed of light
How a Nuclear Plant Works:
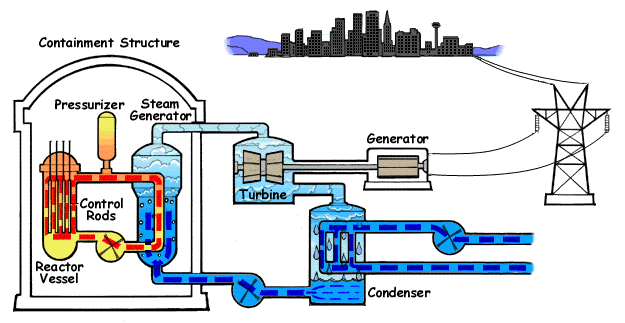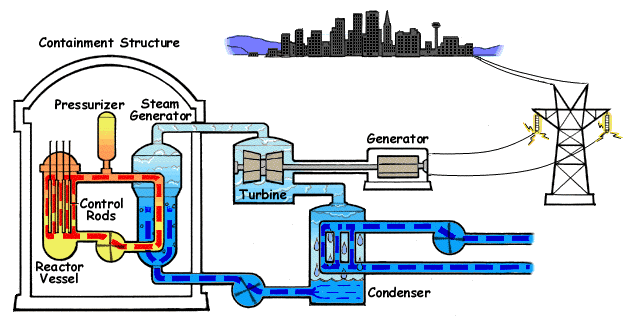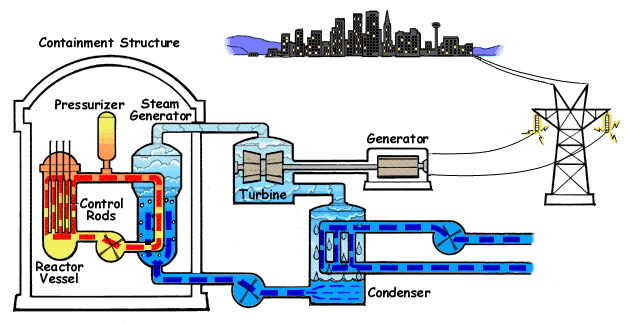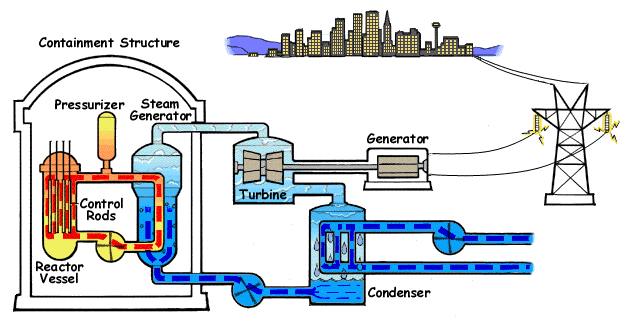
- Uranium fuel is loaded into a reactor. This produces neutrons, splits atoms which makes chain readions. The control rods are used to control the speed of the reaction. Water is pumped in to this. The heated water passes through the heat exchanger. The heat exchanger in turn moves heated water towards the turbine. The turbine is linked to a generator When it turns, the generator does too. The generator turns and produces electricity. This is transported through power grids.
- Apart from Fission, Nuclear Fusion can be used as a power source too: fusion is the process of two or more. nuclei combining to a create a nucleus of greater mass. Example:
${ }_1^2 \mathrm{H}+{ }_1^3 \mathrm{H} \rightarrow{ }_2^4 \mathrm{He}+{ }_0^1 n \rightarrow$ Can do fission
Deuterium Tritium = Isotopes of Hydrogen
- Since the two isotopes are positive, they repel. Hence, a temperate $\&$ of 150 million $० C$ is needed. fission can be used to carry this out.
- Fusion has faults, such as high temperatures, high costs and uncontrollable reactions.
For the amount of heat energy:
$Q=m c \Delta T$
Q=Amount of neat energy
M=mass
c=Specific Heat Capacity
T=change in temperature
- For the amount of heat energy:
$P=\frac{Q}{t}$
P=Power
Q=Amount of heat energy
t=Time
$\begin{array}{r}\text { and } \\ P=\frac{m c \Delta T}{t}\end{array}$
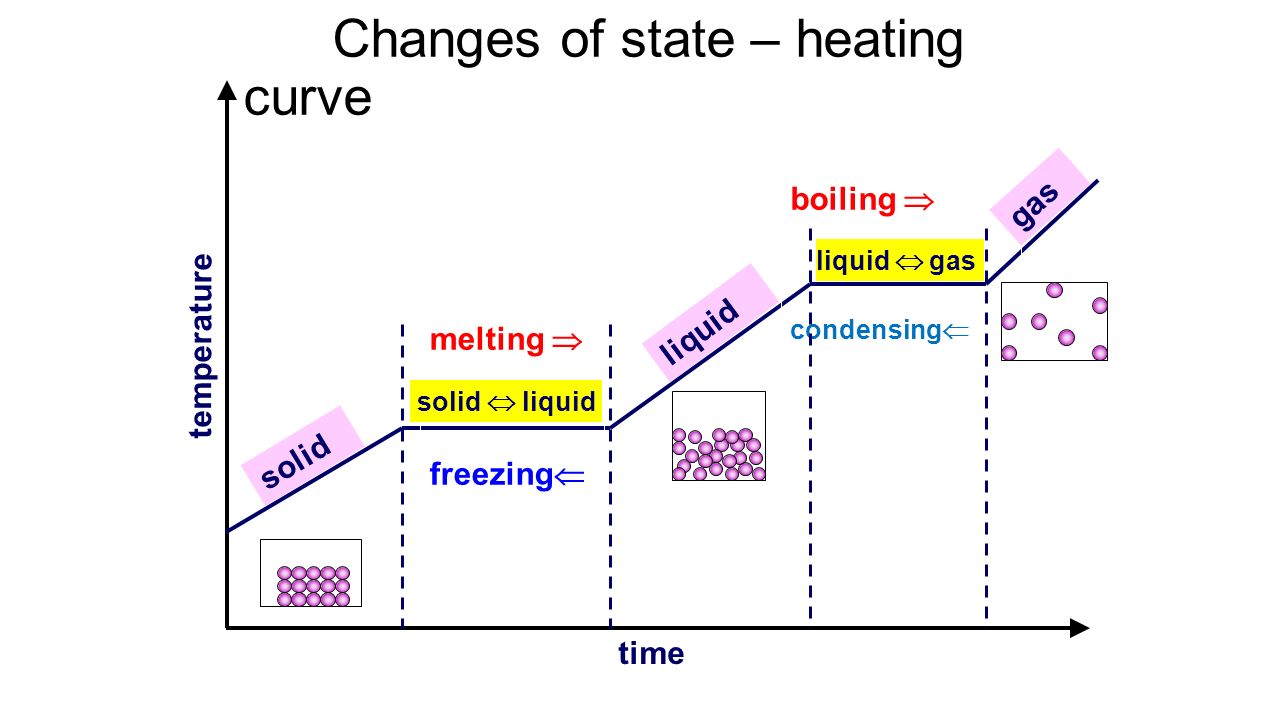
The graph for changes of state: