Question
Aspirin is one of the most widely used drugs in the world.
Aspirin was synthesized from $2.65 \mathrm{~g}$ of salicylic acid (2-hydroxybenzoic acid) $\left(M_{\mathrm{r}}=138.13\right)$ and $2.51 \mathrm{~g}$ of ethanoic anhydride $\left(M_{\mathrm{r}}=102.10\right)$.
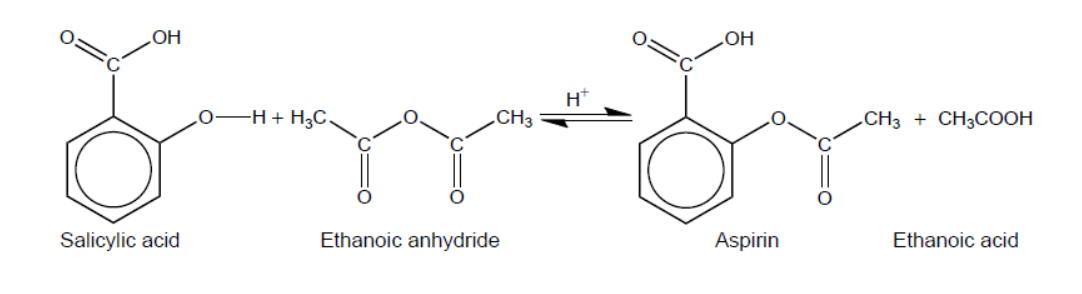
a.iiiSuggest two absorbances, other than the absorbances due to the ring structure and C-H bonds, that would be present in the infrared (IR) spectrum of aspirin.
a.ivState two techniques, other than IR spectroscopy, which could be used to confirm the identity of aspirin.
▶️Answer/Explanation
Markscheme
a.iiiAny two of:
1700-1750 «cm ${ }^{-1} » /$ «absorbance» due to $\mathrm{C}=\mathrm{O}$ in carboxyl/ethanoate
1050-1410 « $\mathrm{cm}^{-1} » /$ «absorbance» due to $\mathrm{C}-\mathrm{O}$ bond in carboxyl/ethanoate
Accept “carboxylic acid” for “carboxyl”, “acetate/ester” for “ethanoate”.
Accept specific wavenumber once within indicated range.
Do not award mark if reference is made to an alcohol/ether.
[2 marks]
a.ivAny two of:
melting point
mass spectrometry/MS
high-performance liquid chromatography/HPLC
NMR/nuclear magnetic resonance
X-ray crystallography
elemental analysis
Accept “spectroscopy” instead of “spectrometry” where mentioned but not “spectrum”.
Accept “ultraviolet «-Visible» spectroscopy/UV/UV-Vis”.
Do not accept “gas chromatography/GC”.
Accept “thin-layer chromatography/TLC” as an alternative to “HPLC”.
[2 marks]
Question
Ibuprofen and paracetamol are mild analgesics. One of the IR spectra below belongs to ibuprofen and the other to paracetamol. The structures of both compounds are given in section 37 of the data booklet.
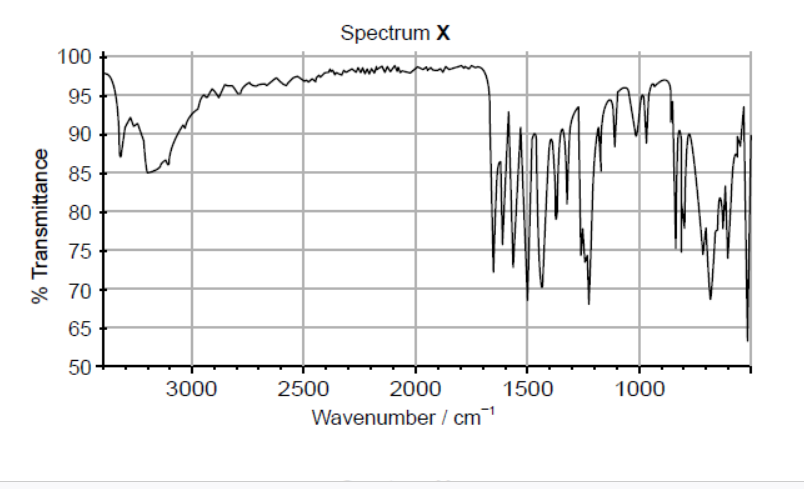
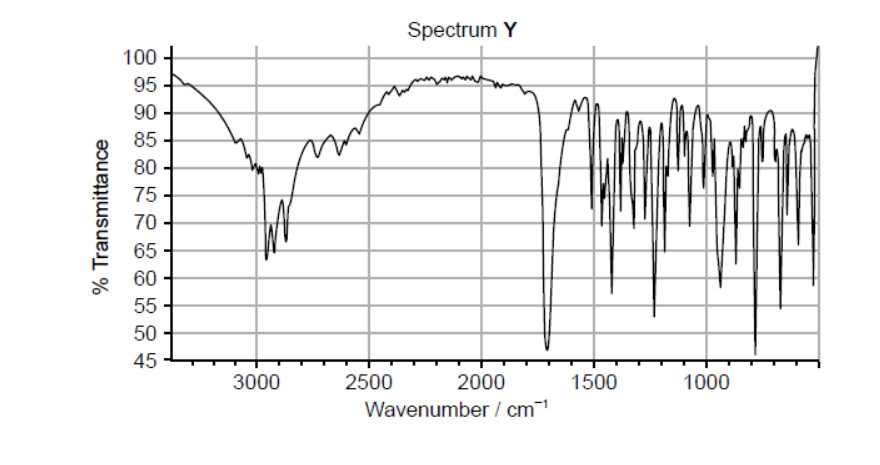
a.i. Both spectra show a peak at wavenumber $1700 \mathrm{~cm}^{-1}$. Identify the bond responsible for this peak.
a.ii.Deduce which spectrum belongs to paracetamol, giving two reasons for your choice. Use section 26 of the data booklet.
b. Describe how mild analgesics function.
▶️Answer/Explanation
Markscheme
a.i. $\mathrm{C}=\mathrm{O}$
Accept “carbonyl”.
a.iiX (must be identified) $\boldsymbol{A N D}$
Any two of:
For $\mathbf{X}$ :
$\mathrm{N}-\mathrm{H}$ «absorption» $A N D$ at $3300-3500 \ll \mathrm{cm}^{-1} » \boldsymbol{V}$
O-H «absorption» in phenol $\boldsymbol{A N D}$ at $3200-3600$ «cm$^{-1} »$
absence of $\mathrm{OH}$ «absorption» in carboxylic acid $A N D 2500-3000$ «cm ${ }^{-1}$ »
Accept any specific wavenumber in the range $3300-3380$ “cm $^{-1} »$ for M1.
Accept any specific wavenumber in the range 3100-3200 «cm ${ }^{-1} »$.
Award [1 max] if $\mathbf{Y}$ is incorrectly identified for paracetamol but if a correct reason/reasons is/are given for the bond absorption(s).
[Max 2 Marks]
b. prevents/interferes with the production of prostaglandins
OR
prevents/interferes with the production of substances responsible for inflammation/pain/fever at the site of injury/source of pain
