Question
(a) Sketch the mechanism for the reaction of 2-methylbut-2-ene with hydrogen bromide using curly arrows. [3]
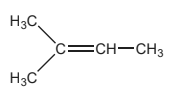
(b) Explain why the major organic product is 2-bromo-2-methylbutane and not 2-bromo-3-methylbutane. [2]
Answer/Explanation
Ans
a.
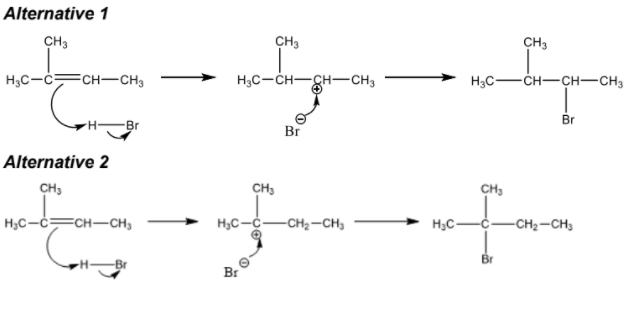
curly arrow going from C=C to H of HBr AND curly arrow showing Br leaving representation of carbocation curly arrow going from lone pair/negative charge on Br − to C+
b
«2-bromo-2-methylbutane involves» formation of more stable «tertiary» carbocation/intermediate
OR «2-bromo-3-methylbutane involves» formation of less stable «secondary» carbocation/intermediate
«intermediate» more stable due to «increased positive» inductive/electron- releasing effect of extra –R/alkyl group/–CH3/methyl
Do not award marks for quoting Markovnikov’s rule without any explanation.
Question
But-1-ene and 1-aminobutane (1-butylamine) can both be prepared from 1-bromobutane.
2-bromobutane and 2-bromo-2-methylpropane are two isomers of 1-bromobutane.
Identify the type of reaction and explain the mechanism for the preparation of but-1-ene from 1-bromobutane using curly arrows to represent the movement of electron pairs.[3]
State the equation (using structural formulas) for the preparation of 1-aminobutane from 1-bromobutane. State the necessary reagents and conditions of the reaction.[3]
Explain the mechanism for the preparation of 1-aminobutane from 1-bromobutane using curly arrows to represent the movement of electron pairs.[4]
Draw the structures of the two mirror images of the isomer that can exhibit optical isomerism.[2]
Describe how the two optical isomers can be distinguished practically using plane-polarized light.[2]
Explain why the mechanism of the reaction will be different if 1-bromobutane is replaced by 2-bromo-2-methylpropane to form 2-amino-2-methylpropane in the reaction in part (a) (iv).[3]
Answer/Explanation
Answer/Explanation
Markscheme
elimination reaction ;
Then accept either E1 or E2 mechanism.
E1
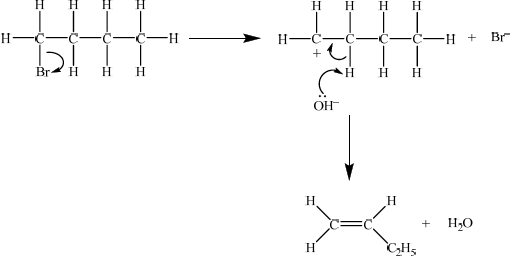
curly arrow showing bromine leaving the halogenoalkane;
\({\text{O}}{{\text{H}}^ – }\) acting as base on the intermediate carbocation;
E2
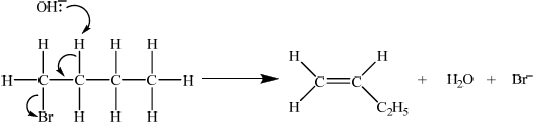
curly arrow showing\({\text{O}}{{\text{H}}^ – }\) acting as base on H bonded to C;
concerted curly arrows showing Br leaving C–Br;
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Br}} + {\text{N}}{{\text{H}}_3} \to {\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2} + {\text{HBr}}\);
ammonia/\({\text{N}}{{\text{H}}_{\text{3}}}\);
warm / excess ammonia (to prevent secondary amines etc.);
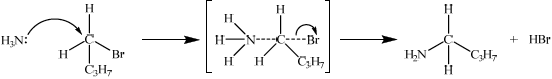
curly arrow from ammonia (to form transition state);
correct transition state;
curly arrow from bond to Br atom in either the first or second step;
formation of HBr and organic product;
Accept a second molecule of NH3 removing H+ from the transition state to give NH4+ and Br– as products.
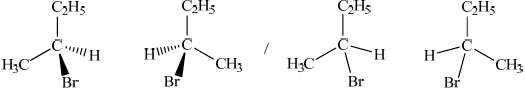
Award [1] for correct structure and [1] for correct 3-D representation of both enantiomers.
polarimeter (to measure angle of rotation);
the plane of plane-polarized light rotates in opposite directions (by the different enantiomers);
2-bromo-2-methylpropane is tertiary / 1-bromobutane is primary;
2-bromo-2-methylpropane goes by SN1 / 1-bromobutane by \({{\text{S}}_{\text{N}}}{\text{2}}\);
intermediate carbocation more stable for tertiary;
no space around tertiary carbon for five groups (in \({{\text{S}}_{\text{N}}}{\text{2}}\) transition state);
Examiners report
This question was the least popular of the Section B questions. Some candidates were very well prepared and scored well, while many struggled to write correct mechanisms with curly arrows in the right place. For a small number of candidates, all parts of the question other than the identification of a functional group proved very difficult. Additionally, in part (a), few candidates knew the details of the reagents and conditions for a range of reaction types.
Question
Iron rusts in the presence of oxygen and water. Rusting is a redox process involving several steps that produces hydrated iron(III) oxide, \({\text{F}}{{\text{e}}_{\text{2}}}{{\text{O}}_{\text{3}}} \bullet {\text{n}}{{\text{H}}_{\text{2}}}{\text{O}}\), as the final product.
The half-equations involved for the first step of rusting are given below.
Half-equation 1: \({\text{Fe(s)}} \to {\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ – }\)
Half-equation 2: \({{\text{O}}_{\text{2}}}{\text{(aq)}} + {\text{4}}{{\text{e}}^ – } + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{4O}}{{\text{H}}^ – }{\text{(aq)}}\)
(i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason for your answer.
(ii) Identify the oxidation number of each atom in the three species in half-equation 2.
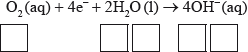
(iii) Deduce the overall redox equation for the first step of rusting by combining half-equations 1 and 2.
(iv) Identify the reducing agent in the redox equation in part (iii).[5]
The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very small concentrations. Explain, in terms of intermolecular forces, why oxygen is not very soluble in water.[2]
State the relationship between the electron arrangement of an element and its group and period in the periodic table.[2]
Transition metals and their compounds often catalyse reactions. The catalyzed decomposition of hydrogen peroxide by CuO is an example. State two other examples of catalyzed reactions giving the transition metal or its compound acting as catalyst.[2]
(i) State a chemical equation for the partial dissociation of water into ions, including state symbols.
(ii) The dissociation of water into ions is reversible. State the expression for the ionic product constant of water.
(iii) The ionic product constant of water was measured at three different temperatures.
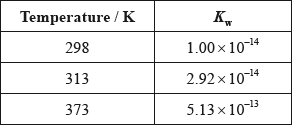
Deduce whether the ionization of water is exothermic or endothermic, giving your reason.
(iv) Use the data in part (iii) to determine the pH of water at 373 K, correct to two decimal places.[6]
(i) An aqueous solution of sodium chloride is electrolysed using inert electrodes. Explain which product is obtained at the positive electrode (anode) if the concentration of sodium chloride is high.
(ii) State the half-equations occurring at the electrodes during the electrolysis of the concentrated aqueous solution of sodium chloride.
Negative electrode (cathode):
Positive electrode (anode):[5]
Describe how electrolysis can be used to electroplate a bracelet with a layer of silver metal. Include the choice of electrodes and electrolyte needed in your description.[3]
Answer/Explanation
Markscheme
(i) oxidation and (iron/Fe) loses electrons/increases in oxidation number/state;
(ii) 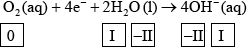 ;
;
Award [2] for five correct.
Award [1] for four correct.
Accept use of oxidation states (0, +1, –2, –2, +1) for oxidation numbers.
Penalize once for incorrect notation (eg, 2, 2–).
(iii) \({{\text{O}}_2}{\text{(aq)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2Fe(s)}} \to {\text{2F}}{{\text{e}}^{2 + }}{\text{(aq)}} + {\text{4O}}{{\text{H}}^ – }{\text{(aq)}}\);
Ignore state symbols.
(iv) Fe/iron;
needs to break strong hydrogen bonds/H–bonds between water molecules (to dissolve) / oxygen cannot form hydrogen bonds/H–bonds with water;
oxygen can only form (weak) van der Waals’/vdW/LDF/London/dispersion forces with water;
groups indicate the number of electrons in the highest energy level/outer/valence shell;
periods indicate the number of (occupied) energy levels/shells (in the atom);
\({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) catalyses oxidation of \({\text{S}}{{\text{O}}_{\text{2}}}\) / \({{\text{V}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) is a catalyst in the Contact Process;
Fe catalyses the reaction between \({{\text{N}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}\) / Fe is a catalyst in the Haber Process;
Ni/Pd/Pt catalyses hydrogenation / manufacture of margarine / addition of hydrogen to C=C / conversion of alkenes to alkanes;
Pd/Pt is a catalyst in catalytic converters / Pd/Pt catalyzes reaction of \({\text{N}}{{\text{O}}_{\text{2}}}\) and CO/\({\text{N}}{{\text{O}}_{\text{2}}}\) and (unburnt) fuel/exhaust gases;
Accept other correct examples.
Accept formulas or names of substances.
(i) \({{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ – }{\text{(aq)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ – }{\text{(aq)}}\);
\( \rightleftharpoons \) and state symbols are necessary for the mark.
(ii) \({K_w} = {\text{[}}{{\text{H}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}/{K_w} = {\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{][O}}{{\text{H}}^ – }{\text{]}}\);
(iii) at higher temperatures ionization increases / at higher temperatures equilibrium shifts to right;
ionization is endothermic;
Do not allow ECF for M2.
(iv) \({\text{5.13}} \times {\text{1}}{{\text{0}}^{ – 13}} = {{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}^2}/{{\text{[}}{{\text{H}}^ + }{\text{]}}^2}/{\text{[}}{{\text{H}}_3}{{\text{O}}^ + }{\text{]}}/{\text{[}}{{\text{H}}^ + }{\text{]}} = {\text{7.16}} \times {\text{1}}{{\text{0}}^{ – 7}}{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{)}}\);
\({\text{pH}} = 6.14/6.15\);
Award [2] for correct final answer.
(i) chlorine/\({\text{C}}{{\text{l}}_{\text{2}}}\) (is produced at the positive electrode/anode);
according to electrochemical series/ \(E^\circ \) values/ease of oxidation \({\text{O}}{{\text{H}}^ – }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released / OWTTE / at low chloride concentration \({\text{O}}{{\text{H}}^ – }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) reacts/oxygen is released;
high concentration makes \({\text{C}}{{\text{l}}^ – }\) oxidize/react in preference to \({\text{O}}{{\text{H}}^ – }\)/\({{\text{H}}_{\text{2}}}{\text{O}}\) / OWTTE;
(ii) Negative electrode (cathode):
\({\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ – } \to {{\text{H}}_2}{\text{(g)}}/{{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ – } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}}/{\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ – } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ – }{\text{(aq)}}\);
Positive electrode (anode):
\({\text{2C}}{{\text{l}}^ – }{\text{(aq)}} \to {\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ – }/{\text{C}}{{\text{l}}^ – }{\text{(aq)}} \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}} + {{\text{e}}^ – }/{\text{2C}}{{\text{l}}^ – }{\text{(aq)}} – {\text{2}}{{\text{e}}^ – } \to {\text{C}}{{\text{l}}_2}{\text{(g)}}/\)
\({\text{C}}{{\text{l}}^ – }{\text{(aq)}} – {{\text{e}}^ – } \to \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\);
Ignore state symbols.
Accept e instead of e–.
Award [1] if half-equations are correct but placed at the wrong electrodes.
bracelet/object to be electroplated is the cathode/negative electrode;
silver anode/positive electrode;
Accept Pt anode.
Electrolyte: liquid \({\text{Na[Ag(C}}{{\text{N}}_{\text{2}}}{\text{)]}}\)/sodium dicyanoargentate/\({{\text{[Ag(CN}}{{\text{)}}_{\text{2}}}{\text{]}}^ – }\)/ solution of an appropriate silver salt;
Accept AgNO3/silver nitrate.
All marks can be scored with a labelled diagram.
Examiners report
(i) Very well answered.
(ii) Most candidates answered correctly. The most common mistakes were doubling the oxidation number of H in \({{\text{H}}_{\text{2}}}{\text{O}}\), and entering a wrong oxidation number for elemental oxygen.
(iii) A well-answered question.
The aqueous solubility of oxygen gas was often poorly explained, with the discussion focussing on the intermolecular forces found in each substance separately and then stating that “like dissolves like”.
Well answered by most candidates.
The majority of candidates were able to give two valid examples of transition metals or their compounds acting as catalysts.
(i) Very well answered.
(ii) Well answered.
(iii) About half of the candidates were able to gain full marks. Some candidates found difficulty in connecting the increase in \({K_{\text{w}}}\) to the position of equilibrium.
(iv) About half of the candidates were able to calculate the pH from the \({K_{\text{w}}}\) value.
(i) Many candidates identified chlorine as the product, but the other two marks were more discriminating. Some candidates clarified that \({\text{C}}{{\text{l}}^ – }\) was oxidized in preference to OH- because of its high concentration, but very few related the situation to the electrochemical series.
(ii) This was poorly answered by many candidates. Common mistakes included releasing sodium at the cathode, reversing electrodes and unbalanced redox half-reactions where the electrons were sometimes on the wrong side of the equation.
Very well answered. Most candidates determined both electrodes correctly. The main difficulty for some candidates was choosing a suitable electrolyte.
