Question
1-bromobutane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}}\), can be converted to 1-aminopentane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{N}}{{\text{H}}_{\text{2}}}\), in a two-step process.
\[\begin{array}{*{20}{l}} {{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{Br}}\xrightarrow{I}{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CN}}} \\ {{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CN}}\xrightarrow{{II}}{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{N}}{{\text{H}}_2}} \end{array}\]
What are the reagents I and II?
▶️Answer/Explanation
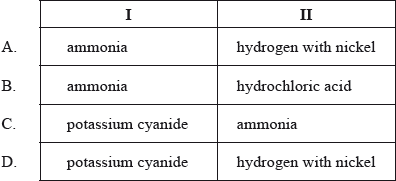
D
To convert 1-bromobutane to 1-aminopentane, you can follow these two steps:
Step 1: Formation of nitrile (1-pentanonitrile)
React 1-bromobutane with potassium cyanide (KCN) to form 1-pentanonitrile.
1-bromobutane + KCN -> 1-pentanonitrile
Step 2: Reduction of nitrile to primary amine (1-aminopentane)
Hydrogenate 1-pentanonitrile using hydrogen gas (H2) in the presence of a nickel catalyst to obtain 1-aminopentane.
1-pentanonitrile + H2 (in the presence of Ni catalyst) -> 1-aminopentane
Question
Which is correct for the conversion of propanal to propyl methanoate?
▶️Answer/Explanation
D
Step 1: Reduction of propanal to propanol
React propanal with sodium borohydride (NaBH4) in the presence of a suitable solvent (such as ethanol or methanol) to reduce propanal to propanol.
Propanal + NaBH4 -> Propanol
It is a reduction reaction.
Step 2: Esterification of propanol with methanoic acid in the presence of concentrated sulfuric acid as a catalyst
React propanol with methanoic acid (formic acid) in the presence of concentrated sulfuric acid as a catalyst to form propyl methanoate (methyl propionate).
Propanol + Methanoic acid (Formic acid) -> Propyl Methanoate (Methyl Propionate).
In the esterification process, an alcohol reacts with a carboxylic acid (or an acid derivative) to form an ester. This reaction involves the nucleophilic substitution of the hydroxyl group (-OH) of the alcohol with the leaving group of the carboxylic acid (usually the -OH group itself or a derivative such as an acid chloride).
The lone pair of electrons on the oxygen atom of the alcohol (the nucleophile) attacks the carbonyl carbon of the carboxylic acid, displacing the leaving group (usually -OH).This leads to the formation of a tetrahedral intermediate. The tetrahedral intermediate loses a water molecule(condensation), resulting in the formation of the ester.
Question
In which order should the reagents be used to convert benzene into phenylamine (aniline)?
▶️Answer/Explanation
C
To convert benzene into phenylamine (aniline), you can follow these three steps:
Step 1: Nitration of benzene to nitrobenzene
React benzene with a mixture of concentrated sulfuric acid and concentrated nitric acid (known as the nitration mixture) to form nitrobenzene.
Benzene + Nitration mixture -> Nitrobenzene
Step 2: Reduction of nitrobenzene to phenylhydroxylamine
React nitrobenzene with tin and hydrochloric acid (HCl) to reduce it to phenylhydroxylamine.
Nitrobenzene + Tin + HCl -> Phenylhydroxylamine
Step 3: Conversion of phenylhydroxylamine to phenylamine
React phenylhydroxylamine with sodium hydroxide (NaOH) to convert it to phenylamine (aniline).
Phenylhydroxylamine + NaOH -> Phenylamine
Question
Propene is reacted first with hydrogen chloride to produce X which is then reacted with aqueous sodium hydroxide to give Y. Finally, Y is reacted with excess acidified potassium dichromate solution.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHC}}{{\text{H}}_{\text{2}}}\xrightarrow{{{\text{HCL}}}}{\text{X}}\xrightarrow{{{\text{NaOH(aq)}}}}{\text{Y}}\xrightarrow{{{{\text{H}}^ + }/{\text{C}}{{\text{r}}_2}{{\text{O}}_7}^{2 – }{\text{(aq)}}}}{\text{Z}}\]
What is the major product, Z?
A. CH3CH(OH)CH3
B. CH3COCH3
C. CH3CH2CHO
D. CH3(CH2)2COOH
▶️Answer/Explanation
B
Step 1: Reaction with Hydrogen Chloride (HCl)
Propene reacts with hydrogen chloride (HCl) to form 2-chloropropane (also known as isopropyl chloride).
Propene + HCl -> 2-Chloropropane
X is 2-Chloropropane.
Step 2: Reaction with Aqueous Sodium Hydroxide (NaOH)
2-Chloropropane undergoes nucleophilic substitution with aqueous sodium hydroxide (NaOH) to form propan-2-ol (isopropyl alcohol).
2-Chloropropane + NaOH -> Propan-2-ol (Isopropyl alcohol)
Y is Propan-2-ol (Isopropyl alcohol).
Step 3: Reaction with Acidified Potassium Dichromate Solution (K2Cr2O7/H2SO4)
Propan-2-ol (isopropyl alcohol) is oxidized by acidified potassium dichromate solution (K2Cr2O7/H2SO4) to form acetone.
Propan-2-ol (Isopropyl alcohol) + Acidified Potassium Dichromate Solution -> Acetone
Z is Acetone. CH3COCH3.
