Question
Graphs showing the first ionization energy and first electron affinity of the elements in period 2 of the periodic table are shown.

(a) Outline why ionization energies have positive values but most electron affinities have negative values.
(b) First ionization energy tends to increase across the period. Explain the decrease in first ionization energy from beryllium to boron.
(c) The electron affinity of nitrogen is 6.8kJ \(mol^{-1}\). Sketch the 2s and 2p orbital filling diagram that represents the electron arrangement of the species produced.
(d) Suggest one reason for a positive value for the first electron affinity for nitrogen.
(e) Suggest reasons why noble gases have the largest first ionization energy and largest positive first electron affinity in their period.
Largest first ionization energy:
Largest positive first electron affinity:
(f) Suggest, giving one reason, how the first electron affinity of xenon compares with that of neon.
Answer/Explanation
Answer:
(a) ionization energy breaks bond/attractive force between nucleus and electron
AND
electron affinity forms bond/attractive force between nucleus and electron
(b) electron «removed» from 2p in B AND 2s in Be
shielding effect of 2s «reduces energy needed to remove 2p»
OR
2p at higher energy level/further from nucleus
OR
full 2s more stable «than single electron in p»
(c) 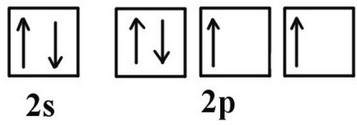
(d) greater repulsion when electrons occupy same orbital
(e) Largest first ionization energy:
highest nuclear charge «for a similar radius/same energy level»
OR
smallest radius
OR
stable octet
Largest positive first electron affinity:
adding to new principal energy level
OR
«much» further from nucleus
OR
shielded by completed inner shell
(f) ALTERNATIVE 1:
«Xe» lower/smaller/less positive AND larger radius
OR
«Xe» lower/smaller/less positive AND smaller energy gap to next «principal»
energy level
ALTERNATIVE 2:
«Xe» greater AND very high nuclear charge
OR
«Xe » greater AND poor shielding by inner «d» orbitals
Question
There has been significant growth in the use of carbon nanotubes, CNT.
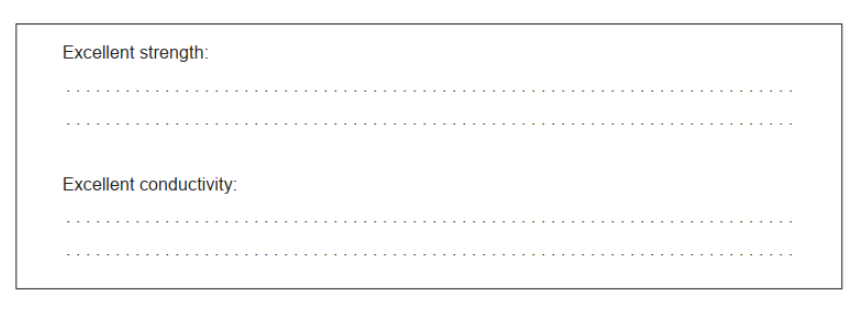
a. Explain these properties of carbon nanotubes.
b(i)Alloying metals changes their properties. Suggest one property of magnesium that could be improved by making a magnesium-CNT alloy.
b(iipure magnesium needed for making alloys can be obtained by electrolysis of molten magnesium chloride.
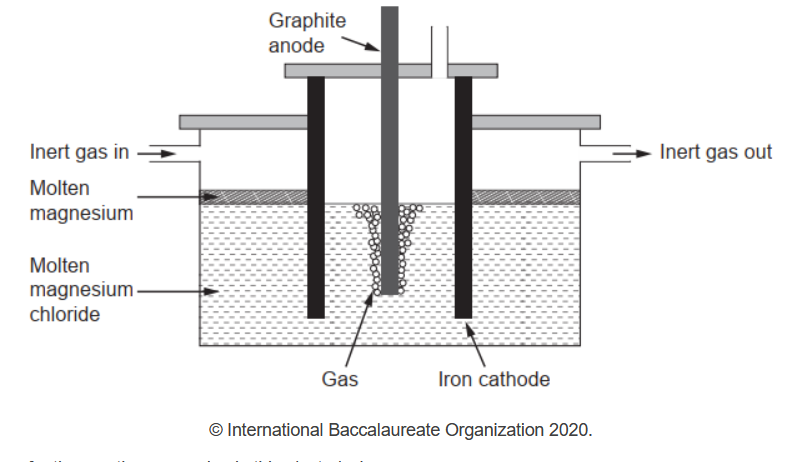
Write the half-equations for the reactions occurring in this electrolysis.
$\mathrm{b}$ (iiifalculate the theoretical mass of magnesium obtained if a current of $3.00 \mathrm{~A}$ is used for 10.0 hours. Use charge $(Q)=\operatorname{current}(I) \times \operatorname{time}(t)$ and section 2 of the data booklet
b(iks.uggest a gas which should be continuously passed over the molten magnesium in the electrolytic cell.
c. Zeolites can be used as catalysts in the manufacture of CNT. Explain, with reference to their structure, the high selectivity of zeolites.
d. Experiments have been done to explore the nematic liquid crystal behaviour of CNT. Justify how CNT molecules could be classified as nematic.
▶️Answer/Explanation
Markscheme
a. Excellent strength: defect-free $A N D$ rigid/regular 2D/3D
Excellent conductivity: delocalized electrons
Accept “carbons/atoms are all covalently bonded to each other” for M1.
b(i)Any of:
ductility
strength/resistance to deformation
malleability
hardness
resistance to corrosion/chemical resistance
range of working temperatures
density
Do not accept “conductivity”.
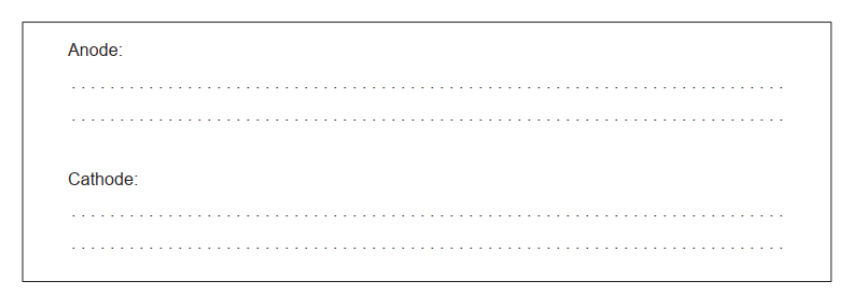
b(ii)Anode: $2 \mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+2 \mathrm{e}^{-}$
Cathode: $\mathrm{Mg}^{2+}+2 \mathrm{e}^{-} \rightarrow \mathrm{Mg}(1)$
Accept $\mathrm{Cl}^{-} \rightarrow \mathrm{Cl}_2(\mathrm{~g})+e^{-}$.
Award [1 max] for correct equations at incorrect electrodes.
$$
\mathrm{b}(\mathrm{iii}) Q=I \times t=3.00 \times 10.0 \times 3600=» 108000 \mathrm{C} \boldsymbol{V}
$$
$$
\begin{aligned}
& « \frac{Q}{F}=\frac{108000 \mathrm{C}}{96500 \mathrm{C} \mathrm{mol}^{-1}}=» 1.12 \ll \mathrm{mol} \mathrm{e}^{-} » \\
& « \frac{1.12 \mathrm{~mol}}{2}=0.560 \mathrm{~mol} \mathrm{Mg} » \\
& « m=0.560 \mathrm{~mol} \times 24.31 \mathrm{~g} \mathrm{~mol}{ }^{-1}=» 13.6 \ll \mathrm{g} »
\end{aligned}
$$
Award [3] for correct final answer.
b(ivergon/Ar/helium/He
Accept any identified noble/inert gas.
Accept name OR formula.
Do not accept “nitrogen $/ \mathrm{N}_2$ “.
c. pores/cavities/channels/holes/cage-like structures
«only» reactants with appropriate/specific size/geometry/structure fit inside/go through/are activated/can react
d. rod-shaped molecules
OR
«randomly distributed but» generally align
OR
no positional order $\boldsymbol{A N D}$ have «some» directional order/pattern
Accept “linear” for “rod-shaped”.