Question
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = – 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}} \end{array}\]
State and explain the effect of increasing the pressure on the yield of sulfur trioxide.[2]
State the effects of a catalyst on the forward and reverse reactions, on the position of equilibrium and on the value of \({K_{\text{c}}}\).[3]
When a mixture of 0.100 mol NO, 0.051 mol \({{\text{H}}_{\text{2}}}\) and 0.100 mol \({{\text{H}}_{\text{2}}}{\text{O}}\) were placed in a \({\text{1.0 d}}{{\text{m}}^{\text{3}}}\) flask at 300 K, the following equilibrium was established.
\(2{\text{NO(g)}} + 2{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + 2{{\text{H}}_2}{\text{O(g)}}\)
At equilibrium, the concentration of NO was found to be \({\text{0.062 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\). Determine the equilibrium constant, \({K_{\text{c}}}\), of the reaction at this temperature.[4]
Outline two differences between an electrolytic cell and a voltaic cell.[2]
Electroplating is an important application of electrolysis. State the composition of the electrodes and the electrolyte used in the silver electroplating process.[3]
Answer/Explanation
Answer/Explanation
Answer/Explanation
Markscheme
yield (of \({\text{S}}{{\text{O}}_{\text{3}}}\)) increases / equilibrium moves to right / more \({\text{S}}{{\text{O}}_{\text{3}}}\) formed;
3 gaseous molecules \( \to \) 2 gaseous molecules / decrease in volume of gaseous molecules / fewer gaseous molecules on right hand side;
Do not allow ECF.
rates of both forward and reverse reactions increase equally;
no effect on position of equilibrium;
no effect on value of [3] \({K_{\text{c}}}\);
\({\text{2NO(g)}} + {\text{2}}{{\text{H}}_2}{\text{(g)}} \rightleftharpoons {{\text{N}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\)
\({\text{[}}{{\text{H}}_{\text{2}}}{\text{] at equilibrium}} = 0.013{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{)}}\);
\({\text{[}}{{\text{N}}_{\text{2}}}{\text{] at equilibrium}} = 0.019{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{)}}\);
\({\text{[}}{{\text{H}}_{\text{2}}}{\text{O] at equilibrium}} = 0.138{\text{ (mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{)}}\);
\({K_{\text{c}}} = {\text{[}}{{\text{N}}_2}{\text{][}}{{\text{H}}_2}{\text{O}}{{\text{]}}^2}{\text{/[NO}}{{\text{]}}^2}{{\text{[}}{{\text{H}}_2}{\text{]}}^2} = {\text{(0.019)(0.138}}{{\text{)}}^2}{\text{/(0.062}}{{\text{)}}^2}{{\text{(0.013)}}^2} = 5.6 \times {10^2}\);
Award [4] for final correct answer.
Accept any value also in range 557–560.
Do not penalize significant figures.
electrolytic cell converts electrical energy to chemical energy and voltaic cell converts chemical energy to electrical energy / electrolytic cell uses electricity to carry out a (redox) chemical reaction and voltaic cell uses a (redox) chemical reaction to produce electricity / electrolytic cell requires a power supply and voltaic cell does not;
electrolytic cell involves a non-spontaneous (redox) reaction and voltaic cell involves a spontaneous (redox) reaction;
in an electrolytic cell, cathode is negative and anode is positive and vice-versa for a voltaic cell / electrolytic cell, anode is positive and voltaic cell, anode is negative / electrolytic cell, cathode is negative and voltaic cell, cathode is positive;
voltaic cell has two separate solutions and electrolytic cell has one solution / voltaic cell has salt bridge and electrolytic cell has no salt bridge;
electrolytic cell, oxidation occurs at the positive electrode/anode and voltaic cell, oxidation occurs at the negative electrode/anode and vice-versa;
Cathode/negative electrode:
object to be plated;
Allow a specific example here e.g. spoon.
Accept inert metal/graphite.
Do not accept silver halides or their formulae.
Anode/positive electrode:
Silver/Ag;
Electrolyte:
\({{\text{[Ag(CN}}{{\text{)}}_{\text{2}}}{\text{]}}^ – }\);
Allow silver nitrate/AgNO3 / silver cyanide/any other suitable silver salt/solution.
Do not accept AgCl.
Examiners report
In (ii) an overwhelming number of candidates were able to score the first mark but did not refer to the gaseous state and hence lost the second mark.
Part (iv) was another question where candidates easily scored the second and third mark. Although this has been asked a number of times in recent sessions, some candidates still do not state that the rates of both the forward and reverse reactions increase equally.
(b) was considered a very challenging question for candidates, and usually only the better candidates scored all four marks.
In (c) (i) most candidates scored two marks.
Electroplating was a topic only partially understood by candidates, and so only a few candidates obtained all three marks in (v). Often the nature of the electrode was mixed up or in many cases incorrect electrolytes were given.
Question
Consider the following reaction studied at 263 K.
\[{\text{2NO(g)}} + {\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2NOCl(g)}}\]
It was found that the forward reaction is first order with respect to \({\rm{C}}{{\rm{l}}_2}\) and second order with respect to NO. The reverse reaction is second order with respect to NOCl.
Consider the following equilibrium reaction.
\[\begin{array}{*{20}{c}} {{\text{C}}{{\text{l}}_2}({\text{g)}} + {\text{S}}{{\text{O}}_2}({\text{g)}} \rightleftharpoons {\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}({\text{g)}}}&{\Delta {H^\Theta } = – 84.5{\text{ kJ}}} \end{array}\]
In a \({\text{1.00 d}}{{\text{m}}^{\text{3}}}\) closed container, at 375 °C, \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}\) and \({\text{8.60}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol}}\) of \({\text{C}}{{\text{l}}_{\text{2}}}\) were introduced. At equilibrium, \({\text{7.65}} \times {\text{1}}{{\text{0}}^{ – 4}}{\text{ mol}}\) of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) was formed.
State the rate expression for the forward reaction.[1]
Predict the effect on the rate of the forward reaction and on the rate constant if the concentration of NO is halved.[2]
1.0 mol of \({\rm{C}}{{\rm{l}}_2}\) and 1.0 mol of NO are mixed in a closed container at constant temperature. Sketch a graph to show how the concentration of NO and NOCl change with time until after equilibrium has been reached. Identify the point on the graph where equilibrium is established.[4]
Consider the following reaction.
\[{\text{N}}{{\text{O}}_2}{\text{(g)}} + {\text{CO(g)}} \to {\text{NO(g)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\]
Possible reaction mechanisms are:
\(\begin{array}{*{20}{l}} {{\text{Above 775 K:}}}&{{\text{N}}{{\text{O}}_2} + {\text{CO}} \to {\text{NO}} + {\text{C}}{{\text{O}}_{\text{2}}}}&{{\text{slow}}} \\ {{\text{Below 775 K:}}}&{{\text{2N}}{{\text{O}}_2} \to {\text{NO}} + {\text{N}}{{\text{O}}_{\text{3}}}}&{{\text{slow}}} \\ {}&{{\text{N}}{{\text{O}}_3} + {\text{CO}} \to {\text{N}}{{\text{O}}_2} + {\text{C}}{{\text{O}}_2}}&{{\text{fast}}} \end{array}\)
Based on the mechanisms, deduce the rate expressions above and below 775 K.[2]
State two situations when the rate of a chemical reaction is equal to the rate constant.[2]
Consider the following graph of \(\ln k\) against \(\frac{1}{T}\) for the first order decomposition of \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{4}}}\) into \({\text{N}}{{\text{O}}_{\text{2}}}\). Determine the activation energy in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) for this reaction.
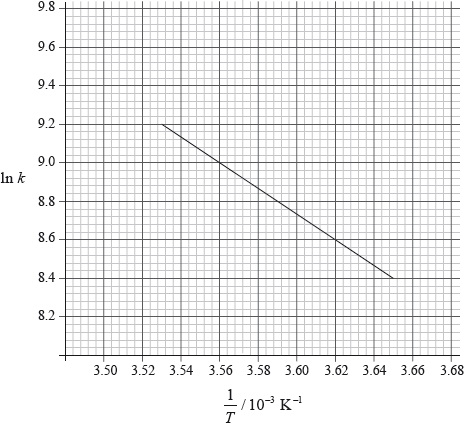 [2]
[2]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.[1]
Determine the value of the equilibrium constant, \({K_{\text{c}}}\).[3]
If the temperature of the reaction is changed to 300 °C, predict, stating a reason in each case, whether the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\) and the value of \({K_{\text{c}}}\) will increase or decrease.[3]
If the volume of the container is changed to \({\text{1.50 d}}{{\text{m}}^{\text{3}}}\), predict, stating a reason in each case, how this will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\) and the value of \({K_{\text{c}}}\).[3]
Suggest, stating a reason, how the addition of a catalyst at constant pressure and temperature will affect the equilibrium concentration of \({\text{S}}{{\text{O}}_{\text{2}}}{\text{C}}{{\text{l}}_{\text{2}}}\).[2]
Answer/Explanation
Answer/Explanation
Answer/Explanation
Markscheme
\({\text{rate}} = k{{\text{[NO]}}^2}{\text{[C}}{{\text{l}}_{\text{2}}}{\text{]}}\);
rate of reaction will decrease by a factor of 4;
no effect on the rate constant;

y axis labelled concentration/\({\text{mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) and x axis is labelled time/s;
gradient for [NO];
gradient for [NOCl] will be equal and opposite;
equilibrium point identified / two curves level off at same time;
Above 775 K: \({\text{rate}} = k{\text{[N}}{{\text{O}}_2}{\text{][CO]}}\);
Below 775 K: \({\text{rate}} = k{{\text{[N}}{{\text{O}}_2}{\text{]}}^2}\);
zero order reaction;
all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\);
\({\text{slope}} = \frac{{9.2 – 8.4}}{{(3.53 – 3.65) \times {{10}^{ – 3}}}} = – 6.67 \times {10^3}\);
\(({E_{\text{a}}} = 6.67 \times {10^3} \times 8.31)\)
\({\text{55.4 (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
Accept in range 55.0 – 56.0
Award [1] if 55454 (J) stated
Award [2] for the correct final answer
\(({K_{\text{c}}}) = \frac{{{\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}}}{{{\text{[C}}{{\text{l}}_2}{\text{][S}}{{\text{O}}_2}{\text{]}}}}\);
Ignore state symbols.
Square brackets [ ] required for the equilibrium expression.
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol of S}}{{\text{O}}_2}\) and \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol of C}}{{\text{l}}_2}\);
\({\text{7.84}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{ of S}}{{\text{O}}_2}\), \({\text{7.84}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{ of C}}{{\text{l}}_2}\) and
\({\text{7.65}} \times {\text{1}}{{\text{0}}^{ – 4}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{ of S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}\);
12.5;
Award [1] for 10.34
Award [3] for the correct final answer
value of \({K_{\text{c}}}\) increases;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) increases;
decrease in temperature favours (forward) reaction which is exothermic;
Do not allow ECF.
no effect on the value of \({K_{\text{c}}}\) / depends only on temperature;
\({\text{[S}}{{\text{O}}_2}{\text{C}}{{\text{l}}_2}{\text{]}}\) decreases;
increase in volume favours the reverse reaction which has more gaseous moles;
Do not allow ECF.
no effect;
catalyst increases the rate of forward and reverse reactions (equally) / catalyst decreases activation energies (equally);
Examiners report
In part (a) the rate expression was correctly stated although some confused this with an equilibrium constant expression.
Only the better candidates realized that the rate of reaction will decrease by a factor of four and there will be no effect on the rate constant.
Although most candidates were able to correctly sketch the concentration versus time graph many forgot to label the axes or include units.
Part (b) was well answered and candidates demonstrated a good understanding of rate expressions based on reaction mechanism.
The better candidates were able to figure out that the rate of a chemical reaction is equal to the rate constant when all concentrations are \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) or for a zero order reaction.
Most candidates had difficulty in calculating activation energy from the graph in part (d) and some gave the answer in \({\text{J}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) instead of \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\) which showed that they missed this instruction in the question.
In part (e), the equilibrium constant expression was correctly stated by the majority but calculating the value of\({K_{\text{c}}}\) proved to be difficult.
A large number of candidates obtained the incorrect answer of 10.34 as a result of using the initial concentrations of the reactants instead of equilibrium concentrations.
[N/A]
The application of Le Chatelier’s principle was handled well by the majority with minor omissions such as not using the term gaseous particles in part (iv).
Some candidates stated that the addition of a catalyst does not affect the value of \({K_{\text{c}}}\) or the position of equilibrium, which did not answer the question and scored no marks because they had not commented on the concentration of \({\text{SOC}}{{\text{l}}_{\text{2}}}\). Some candidates correctly stated that a catalyst increases the rate of forward and reverse reactions equally.
