Question
Sections of two forms of polystyrene are shown:
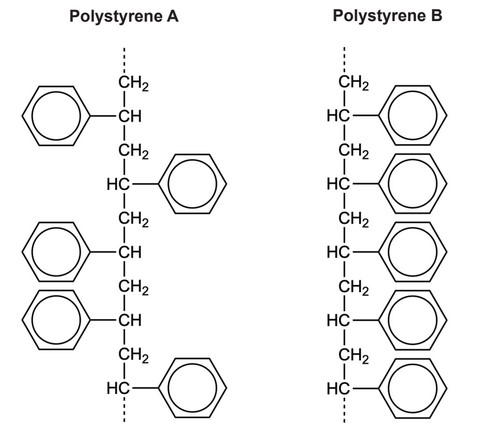
(a) (i) Draw the structural formula of the monomer from which they were formed.
(ii) Identify, giving one reason, the form with the higher melting point.
(b) Explain how a substance in the same phase as the reactants can reduce the activation energy and act as a catalyst.
(c) Solutions of substituted polystyrenes can form lyotropic liquid crystals. Outline how lyotropic liquid crystals differ from other liquid crystals.
(d) Expanded polystyrene (EPS) is a useful material.
(i) Explain how polystyrene is converted to EPS.
(ii) State one property of EPS that makes it a useful material.
Nylon-6 is a polymer that can be formed from the monomer:
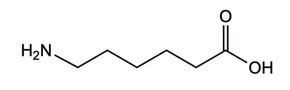
(e) State the type of polymerization reaction that occurs and the structural characteristic of the monomer that allows this type of polymerization to occur.
Type of polymerization:
Structural characteristic:
(f) Outline why plastics do not break down easily in the environment.
(g) State the RIC number for polyamide plastic (nylon). Use section 30 of the data booklet.
Answer/Explanation
Answer:
(a) (i) 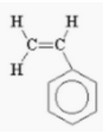
(ii) B AND chains «of polymer» can align/pack more closely
(b) forms an intermediate/activated complex
«intermediate/activated complex» dissociates to form product «AND catalyst»
(c) «lyotropic liquid crystals» exist over a given concentration range AND other liquid
crystals exist over a certain temperature range
(d) (i) volatile hydrocarbon/pentane «incorporated in beads of the polymer»
vaporizes/boils when heated «causing polymer to expand»
(ii) «good» thermal/electrical insulator
OR
soft/provides shock resistance
OR
low density
OR
easily moulded/versatile
OR
water resistant
OR
durable
(e) Type of polymerization: condensation
Structural characteristic: two functional groups
(f) strong covalent bonds
(g) «RIC» 7
Question
Carbon fibre reinforced plastic (CFRP) is a useful composite. Epoxy is a thermoset polymer that is used as a binding polymer when making CFRP.
a. Outline the two distinct phases of this composite.
b(i)Thermoplastic composites are increasingly replacing thermosets.
Suggest one advantage of thermoplastic polymers over thermosets.
b(ii Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the addition of phthalate ester plasticizers.
$b$ (iifæxplain why phthalates are replaced by other plasticizers in the production of plastics.
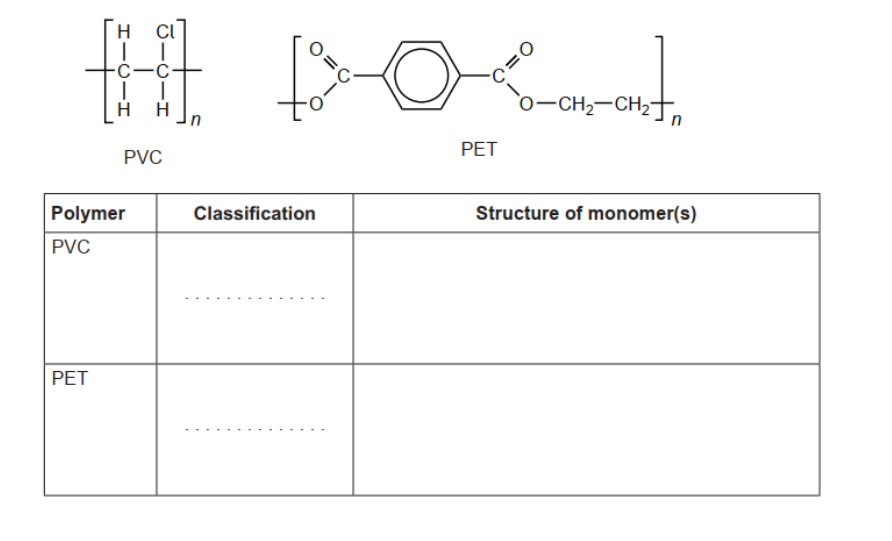
c. Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce the structural formulas.
▶️Answer/Explanation
Markscheme
a. carbon fibre reinforcing phase
«in a» matrix phase of epoxy
Award [1 max] for “reinforcing phase “embedded” in a matrix”.
b(i)can be recycled
OR
can be reformed when hot
OR
high impact/chemical/abrasion resistance
$\mathrm{b}(\mathrm{ii})$ Any three of:
plasticizers embed/fit between «polymer» chains
keep polymer strands/chains/molecules separated/apart
weaken intermolecular/London/dispersion/attractive/forces/instantaneous induced dipole-induced dipole/forces «between chains» prevent chains from packing closely/forming regular packing/structure
Accept “van der Waals/vdW” for “London”.
b(iii)Any two of:
readily released into environment
OR
have weak intermolecular forces «rather than covalent bonds between chains»
get into biological systems by ingestion/inhalation
interrupt endocrine systems
OR
affect release of hormones
OR
effect development of male reproductive system
considered carcinogenic
OR
can cause cellular damage
can cause early puberty in females
can cause thyroid effects
can cause asthma
Do not accept just “are a health concern”.
c.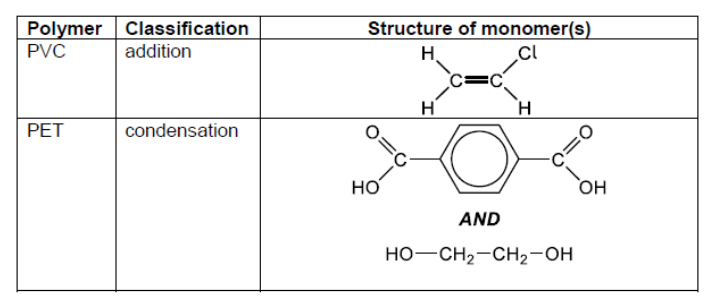
PVC: addition AND PET: condensation structure of PVC monomer structure of PET monomers Accept full OR condensed structural formulas.
