Question
a. Outline two differences between heterogeneous and homogeneous catalysts.
b. Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
c(i)Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
c(ii)The trace quantities of dioxins from tyre fires are rarely inhaled and instead settle on the ground.
Describe why this is a health concern.
▶️Answer/Explanation
Markscheme
a. Any two of:
heterogeneous catalyst is in different phase than reactants AND homogeneous catalyst in same phase [ $\checkmark$ ]
homogeneous catalysts chemically change/react and are reformed at end of reaction
OR
reactants adsorb onto heterogenous catalyst and products desorb [ $\checkmark$ ]
heterogeneous catalysts are more easily removed than homogenous catalysts $[\boldsymbol{V}]$
heterogeneous catalysts can function at higher temperatures $[\boldsymbol{V}]$
homogeneous catalysts are “generally» more selective $[\boldsymbol{\sim}]$
homogeneous catalysts offer a broader range of reactions $[\boldsymbol{V}]$
Note: Accept “state” for “phase”.
Accept “heterogeneous catalyst provides a surface to activate reaction”.
b. elastomers bend under force «and return to original form when force is released»
OR
elastomers make tyre more flexible $[\boldsymbol{V}]$
allows greater contact with road $[\boldsymbol{\omega}$ ]
c(i)does not contain heterocyclic ring with 2 oxygen atoms
OR
middle ring has only 1 oxygen atom [ $\checkmark]$
produces similar toxic effects to dioxins [ $\boldsymbol{V}$ ]
Note: Accept “does not contain dioxin ring” for M1.
c(ii)taken up by plants, which are eaten by animals «and then further dispersed»
OR
passed on in food chain [ $\boldsymbol{V}]$
Note: Accept “do not break down and can be remobilised as dust”.
Question
Polymers have a wide variety of uses but their disposal can be problematic.
a. Draw a section of isotactic polychloroethene (polyvinylchloride, PVC) showing all the atoms and all the bonds of four monomer units.
b. The infrared (IR) spectrum of polyethene is given.
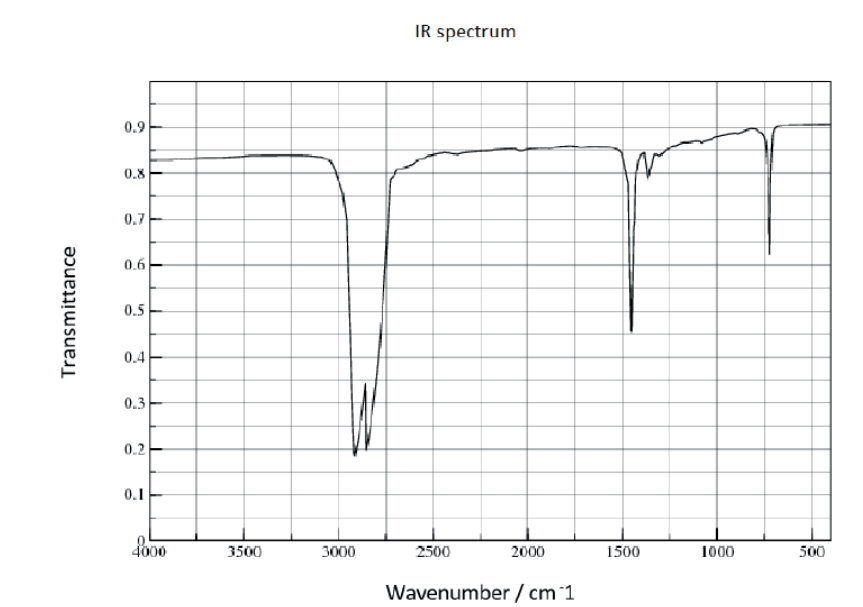
Suggest how the IR spectrum of polychloroethene would differ, using section 26 of the data booklet.
c. Identify a hazardous product of the incineration of polychloroethene.
d. Explain how plasticizers affect the properties of plastics.
e. Suggest why the addition of plasticizers is controversial.
▶️Answer/Explanation
Markscheme
a.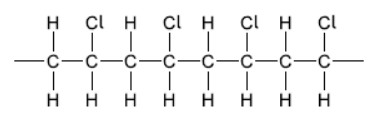
correct bonding [ $\boldsymbol{]}$
$\mathrm{Cl}$ atoms all on same side and alternate [ $\boldsymbol{C}$
Note: Continuation bonds must be shown.
Award [1 max] if less than or more than four units shown.
Accept a stereo formula with all atoms and bonds shown.
b. «strong additional» absorption at $600-800$ «cm ${ }^{-1}$ [ [ ]
c. Any one of:
$\mathrm{HCl}[\boldsymbol{\mu}]$
$\mathrm{Cl}_2[\boldsymbol{\varkappa}]$
dioxins $[\boldsymbol{\sim}]$
C $[\leftarrow]$
$\mathrm{CO}[\boldsymbol{\kappa}]$
d. Any two of:
embedded/fit between chains of polymers [ $\boldsymbol{C}$ ]
keep polymer strands/chains/molecules separated/apart [ $\boldsymbol{\backslash}$ ]
increase space/volume between chains $[\boldsymbol{\nu}]$
weaken intermolecular/dipole-dipole/London/dispersion/instantaneous dipoleinduced dipole/van der Waals/vdW forces «between chains» [ $\boldsymbol{\nu}$ ]
increase flexibility/durability/softness $[\boldsymbol{\swarrow ]}$
make polymers less brittle $[\boldsymbol{\sim}]$
e. leach into foodstuffs/environment
OR
«unknown» health/environmental consequences [ $\sim$ ]
Note: Accept “plasticizers cannot be recycled”.
Question
One way of classifying materials is based on the type of bonding present.
One reaction to convert cyclohexanone to caprolactam using concentrated sulfuric acid as a catalyst is shown.
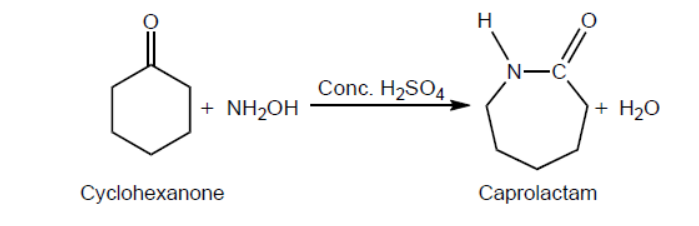
a. Outline why this type of classification is not entirely satisfactory by using magnesium diboride, $\mathrm{MgB}_2$, as an example. Refer to sections 8 and 29 of the data booklet.
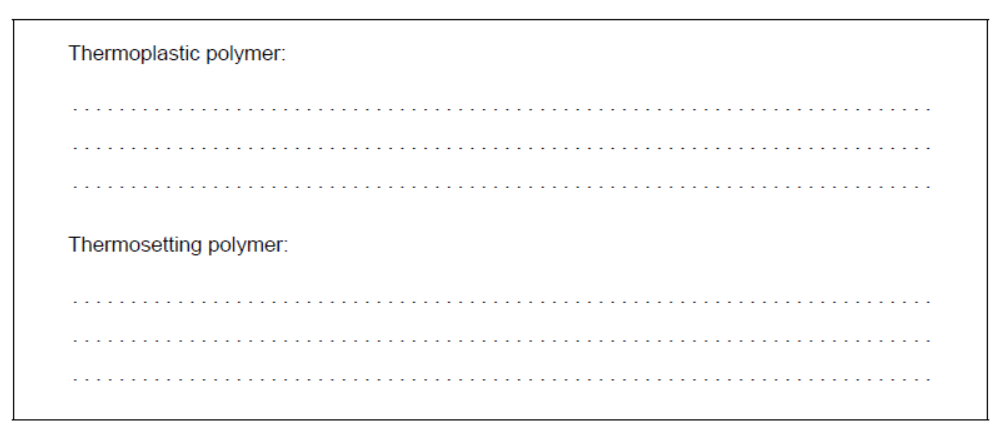
b.i. Structures of poly(methyl acrylate), PMA, and Bakelite ${ }^{\circledR}$ are shown.
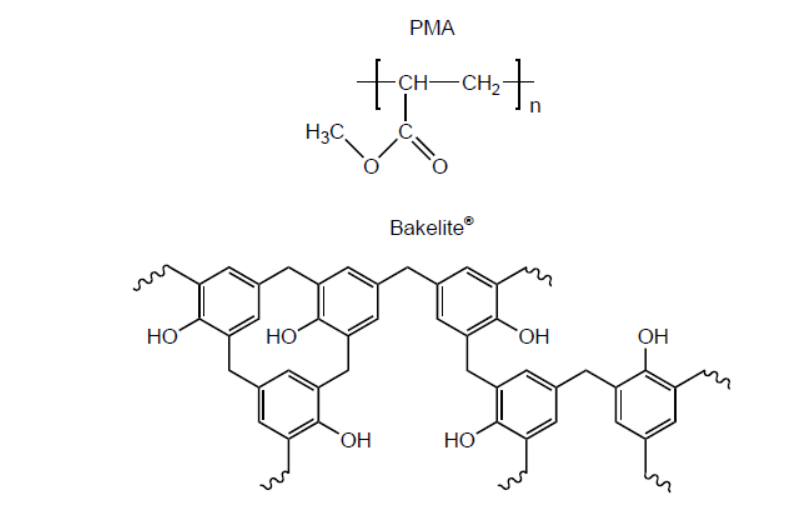
Suggest, giving reasons, which is the thermoplastic polymer and which is the thermosetting polymer.
b.ii.In an incomplete combustion of the polyvinyl chloride, PVC, it was found that hydrogen chloride, carbon monoxide, carbon dioxide, and water vapour were released.
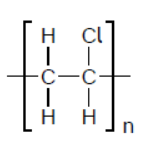
Formulate an equation for this reaction using the formula of the PVC repeating unit.
c.i. A zeolite is an alternative catalyst for this reaction.
Explain how zeolites act as selective catalysts.
c.ii.Identify another advantage of using a zeolite instead of concentrated sulfuric acid.
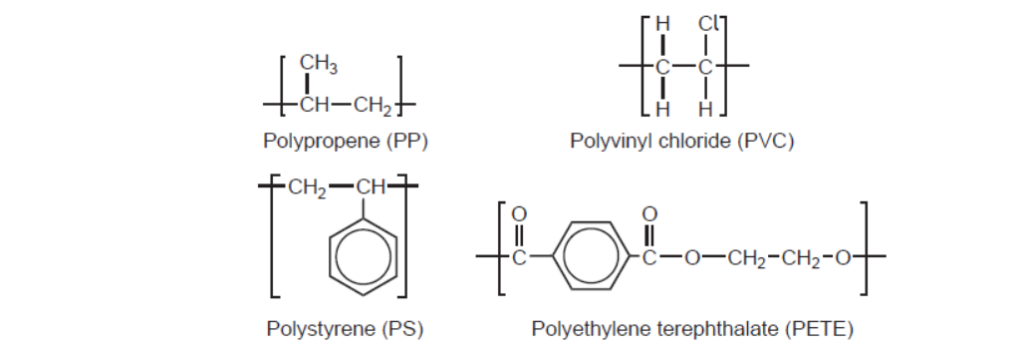
d. Repeating units of several polymers are listed.
The infrared (IR) spectrum of one of these polymers is shown.
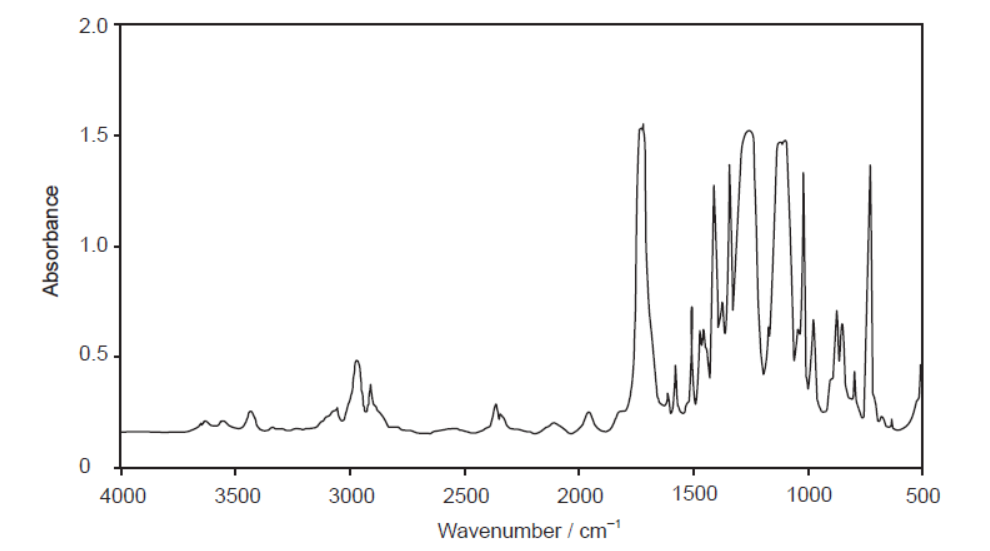
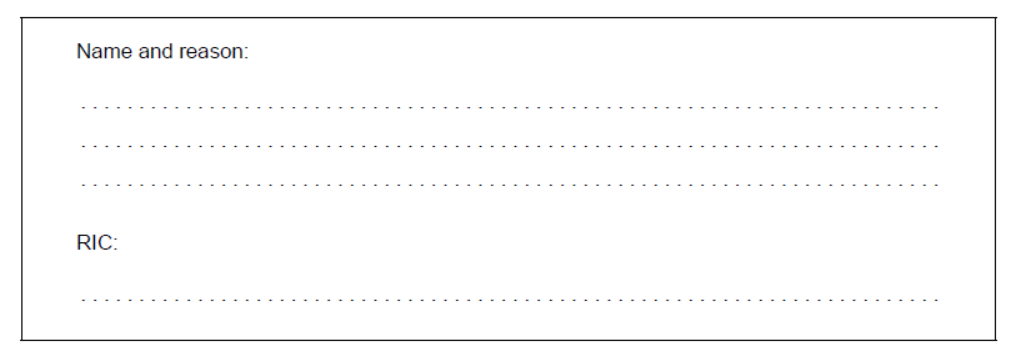
Deduce, giving a reason, the name of this polymer and its Resin Identification Code (RIC), using sections 26 and 30 in the data booklet.
▶️Answer/Explanation
Markscheme
a. $\Delta \chi=0.7$ AND average $\Delta \chi=1.7$
bonding between metallic and ionic
OR
more than one type of bonding present
OR
bond type difficult to determine as close to several regions/several types/named bonding types «eg ionic and covalent etc.»
OR
bond is mostly covalent «based on \% covalent scale on diagram»
OR
bond has « $\frac{0.7}{3.2} \times 100=» 22 \%$ ionic character
Accept “EN” for ” $\chi$ “.
Accept “bond is ionic but close to several regions/several types/other named bonding type(s) (eg covalent, metallic and covalent etc.)”.
Do not accept just “bond is ionic”.
Accept any value for $\%$ ionic character in range $15-24 \%$ or $\%$ covalent character in range $76-85 \%$.
b.i. Thermoplastic polymer:
PMA AND «weak» intermolecular/IMFs/London/dispersion/van der Walls/vdW/dipole-dipole forces «between layers/chains» OR
PMA AND no/few cross-links «between layers/chains»
Thermosetting polymer:
Bakelite ${ }^{\circledR}$ AND «strong» covalent bonds «between layers/chains»
OR
Bakelite ${ }^{\circledR}$ AND extensive cross-links «between layers/chains»
Do not accept “hydrogen bonding” for M1.
Award [1 max] for correct reasons for both polymer classes even if named polymers are incorrectly classified.
b.ii. $\mathrm{CH}_2 \mathrm{CHCl}(\mathrm{s})+2 \mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{HCl}(\mathrm{g})+\mathrm{CO}(\mathrm{g})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})$
OR
$\mathrm{CH}_2 \mathrm{CHCl}(\mathrm{s})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{HCl}(\mathrm{g})+2 \mathrm{CO}(\mathrm{g})+\mathrm{H}_2 \mathrm{O}(\mathrm{g})$ AND $2 \mathrm{CO}(\mathrm{g})+\mathrm{O}_2(\mathrm{~g}) \rightarrow \mathrm{CO}_2(\mathrm{~g})$
Accept any correctly balanced equation that includes the products specified.
c.i. pores/cavities/channels/holes/cage-like structures «in zeolites» have specific shape/size
only reactants «with appropriate size/geometry» fit inside/go through/are activated/can react
c.ii.does not require corrosive acid/«concentrated» sulfuric acid/ $\mathrm{H}_2 \mathrm{SO}_4$
OR
zeolite can be recycled «more easily»
OR
product can be «more» easily separated from a zeolite «than from sulfuric acid»
OR
minimal/less impact on environment
OR
synthesis of specific isomers as products
d. Name and reason:
PET/PETE AND peak for $\mathrm{C}=\mathrm{O}$ «at $1700-1750 \mathrm{~cm}^{-1}$ »
RIC:
1
Accept “PET/PETE AND peak for C-O «at 1050-1410 cm”-1»” for M1.
Accept “PET/PETE AND peak(s) for COO” for M1.
Accept name or abbreviation for polymer.
No ECF for $M 2$.
Question
Landfill sites are used to dispose of about 90% of the world’s domestic waste, but incineration is being increasingly used in some countries.
Suggest why some biodegradable plastics do not decompose in landfill sites.
High-level and low-level wastes are two types of radioactive waste. Compare the half-lives and the methods of disposal of the two types of waste.
▶️Answer/Explanation
Markscheme
limited supply of oxygen (prevents the bacteria from acting);
Do not accept air.
high-level waste has longer half-life / low-level waste has shorter half-life;
high-level waste is vitrified/made into glass/buried underground/in granite/in deep mines/under water/in steel containers/in cooling ponds / OWTTE;
low-level waste is stored under water/in steel containers/in cooling ponds/filtered/discharged directly into sea / OWTTE;
Accept cooling ponds/steel containers/under water/concrete containers only once.
Examiners report
This was generally well done although few realised that oxygen was needed for the decomposition of the plastics in landfill sites.
This was generally well done although few realised that oxygen was needed for the decomposition of the plastics in landfill sites.
Question
Since the accidental discovery of polyethene in the 1930s, polymers have played an essential role in daily life because of their wide range of properties and uses.
Titanium compounds are used as catalysts in the manufacture of high-density polyethene (HDPE). Discuss two factors scientists would have considered in choosing these catalysts.
Describe a structural feature of low-density polyethene (LDPE) that explains why LDPE has a different melting point from that of HDPE.
State one environmental impact of the disposal of these polyethenes by using incineration.
▶️Answer/Explanation
Markscheme
no other product formed (except HDPE);
expensive but effective;
little or no environmental/health impact;
not easily poisoned by impurities;
cause (considerable) increase in rate;
ability to work under mild/severe conditions;
side-chains / branching present (in LDPE);
limits closer packing / chains further apart / OWTTE;
less van der Waals’/dispersion/London forces / OWTTE;
Award mark for less intermolecular forces. Do not accept weaker.
low(er) melting point (than HDPE);
No mark for different melting point.
Accept reverse argument for HDPE.
\({\text{C}}{{\text{O}}_2}\) is a greenhouse gas / causes climate change / global warming / formation of soot/particulates / melting of polar ice caps / rising sea levels / OWTTE;
Accept CO produced is toxic/poisonous.
Examiners report
(a) was surprisingly poorly answered, with very few candidates understanding how to discuss two factors in choosing catalysts, although they should have studied several factors.
(b) was answered well.
(c) was answered well.
Question
The development and application of plastics was one of the most important technological developments of the last century.
The diagram below represents a section of a polymer.
Identify the two functional groups in the monomer from which this polymer is manufactured.
An expanded form of the plastic is often used in packaging. Describe how this is manufactured.
Discuss two advantages and one disadvantage of using the expanded form as a packaging material.
Two advantages:
One disadvantage:
▶️Answer/Explanation
Markscheme
alkenyl/C=C and phenyl/–\({{\text{C}}_{\text{6}}}{{\text{H}}_5}\);
Accept alkene and benzene ring/aromatic ring/arene but not benzene.
pentane/volatile hydrocarbon added (during polymerization process);
heating causes pentane/volatile hydrocarbon to evaporate/vaporize/produce bubbles of gas (expanding the polystyrene);
Accept other suitable identified blowing agents such as carbon dioxide.
Advantages:
Any two for [2 max]:
low/reduced density;
Accept “small mass”.
Do not accept “light”.
can be shaped (around object);
good shock absorber;
insulator;
Disadvantage:
Award [1] for disadvantage:
disposal takes up a lot of space (in landfill);
Accept “non-biodegradable/polluting/hazardous to wildlife”.
Examiners report
Some candidates identified two functional groups in the monomer.
About half of the candidates described how the expanded form of the plastic could be manufactured.
Most candidates were able to score one or two marks. “Good shock absorber” was a popular answer. Candidates should be encouraged to use precise terminology such as “has low density” instead of “light”.
Question
There has been a shift in the use of crude oil (petroleum) away from its use as an energy source and towards its use as a chemical feedstock.
Suggest two reasons for this shift.
A lot of feedstock is used in the production of plastics. Discuss two advantages and one disadvantage of using plastic for packaging instead of cardboard.
Two advantages:
One disadvantage:
▶️Answer/Explanation
Markscheme
increasing cost of oil (relative to other energy sources);
limited supply (of petroleum);
other sources of energy available / alternative energy sources;
(use as a raw material) reduces/delays greenhouse gas/global warming/climate change problems;
concerns about greenhouse gases/climate change causing changes in behaviour / OWTTE;
Do not accept just “greenhouse gases/climate change”;
products from raw materials can be recycled / fuels cannot be recycled;
increasing demand as raw material from continued economic growth/demand for wider variety of products;
more profit to be made (by using as raw material);
reduced availability of other sources of hydrocarbons;
Accept political factors, such as “conflicts disrupting production”.
Advantages:
Any two for [2 max] of:
waterproof so strong when wet;
can be transparent so contents can be seen;
better insulates the item it is packing if expanded plastic/bubble wrap used;
can be vacuum sealed to exclude air/keep food fresh;
better protection against knocks as it can be moulded to fit the item;
Disadvantages:
Any one of:
uses valuable petroleum resources which are non-renewable;
(may) not be burned safely because toxic gases are produced;
(may) not be bio-degradable/recyclable so will linger in landfill;
Accept other valid answers for both advantages and disadvantages.
Each answer must be qualified.
Examiners report
Many candidates did achieve at least one mark, usually referring to the increasing demand of crude oil as a raw material linked to demand for wider variety of products. Any other reasons were often inadequately communicated. There were many responses referring to the ‘production of greenhouse gases’ with no further qualification with respect to the shift in behaviour. The second part of this question produced answers which often failed to precisely address the advantages and disadvantages of the use of plastics versus cardboard specifically for packaging.
Many candidates did achieve at least one mark, usually referring to the increasing demand of crude oil as a raw material linked to demand for wider variety of products. Any other reasons were often inadequately communicated. There were many responses referring to the ‘production of greenhouse gases’ with no further qualification with respect to the shift in behaviour. The second part of this question produced answers which often failed to precisely address the advantages and disadvantages of the use of plastics versus cardboard specifically for packaging.
Question
Chloroethene undergoes polymerization with a free-radical initiator to produce the atactic form of polychlorethene (PVC).
Sketch the atactic form of polychloroethene showing four units.
(i) Explain, in molecular terms, why PVC becomes more flexible and softer when a plasticizer is added.
(ii) State one type of compound which can be used as a plasticizer.
Suggest an environmental issue associated with the use of PVC.
▶️Answer/Explanation
Markscheme
correct structure with random orientation of Cl atoms
Accept 2-dimensional diagrams.
Accept any random arrangement of Cl atoms providing the monomer units originate from chloroethene and Cl atoms are on alternate carbons.
Continuation bonds are necessary for the mark.
(i)
«plasticizer molecules» fit between chains
OR
«plasticizer molecules» prevent chains from forming crystalline regions
OR
«plasticizer molecules» keeps strands/chains/molecules separated
OR
«plasticizer molecules» increase space/volume between chains
weakens intermolecular/dipole-dipole/London/dispersion/instantaneous induced dipole-induced dipole/van der Waals/vdW forces
Do not accept “«plasticizer molecules» lower density”.
(ii)
ester/phthalate/citrate
Accept other general or specific names of plasticizers.
does not degrade/biodegrade/break down «easily»
occupies more space in landfills
incineration produces dioxins/hydrochloric acid/HCl «which can contribute to acid rain»
Accept “plasticizer added to PVC can be a health hazard”.
Accept “combustion” for “incineration”.
Do not accept simply “toxic compounds” for M3
Question
The development of materials with unique properties is critical to advances in industry.
Low density polyethene (LDPE) and high density polyethene (HDPE) are both addition polymers.
Outline two properties a substance should have to be used as liquid-crystal in a liquid-crystal display.
Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
One of the two infrared (IR) spectra is that of polyethene and the other of polytetrafluoroethene (PTFE).
Deduce, with a reason, which spectrum is that of PTFE. Infrared data is given in section 26 of the data booklet.
Many plastics used to be incinerated. Deduce an equation for the complete combustion of two repeating units of PVC, (–C2H3Cl–)2.
▶️Answer/Explanation
Markscheme
Any two of:
ability to form a LC phase
chemically stable
«LC phase that is» stable over suitable temperature range
polar
OR
being able to change orientation with applied electric field
rapid switching speed «responds to changes of voltage quickly»
Accept “ability of molecules to transmit light under certain conditions” OR “rodshaped molecules” OR “stable to light/not light sensitive”.
[Max 2 Marks]
branching in LDPE prevents close packing «of chains»
LDPE is more flexible/less rigid
OR
LDPE has lower «tensile» strength
Do not accept “difference in density”.
Award [1 max] for stating “branching in LDPE AND little/no branching in HDPE”.
B AND absence «of absorption of» C–H at 2850–3090 «cm–1»
OR
B AND presence of «absorption of» C–F at 1000–1400 «cm–1»
(–C2H3Cl–)2 (s) + 5O2 (g) → 4CO2 (g) + 2H2O (l) + 2HCl (g)
correct species in reactants and products
balanced
Accept “(–C2H3Cl–)2 (s) + 5.5O2 (g) → 4CO2 (g) + 3H2O (l) + Cl2 (g)”.
Award M2 only if M1 correct.
Question
Both HDPE (high density polyethene) and LDPE (low density polyethene) are produced by the polymerization of ethene.
Both of these are thermoplastic polymers. Outline what this term means.
Compare and contrast the structures of HDPE and LDPE.
State one way in which a physical property of HDPE, other than density, differs from that of LDPE as a result of this structural difference.
The production of HDPE involves the use of homogeneous catalysts. Outline how homogeneous catalysts reduce the activation energy of reactions.
Trace amounts of metal from the catalysts used in the production of HDPE sometimes remain in the product. State a technique that could be used to measure the concentration of the metal.
Suggest two of the major obstacles, other than collection and economic factors, which have to be overcome in plastic recycling.
Suggest why there are so many different ways in which plastics can be classified. HDPE can, for example, be categorized thermoplastic, an addition polymer, having Resin Identification Code (RIC) 2, etc.
▶️Answer/Explanation
Markscheme
soften/melt when heated
OR
can be melted and moulded
Accept “low melting point” OR “can be moulded when heated”.
[1 mark]
both have «long» hydrocarbon chains
OR
both have chains comprising CH2 units
HDPE has little/no branching AND LDPE has «more» branching
Accept “CH2–CH2 units”.
Accept “HDPE more crystalline”.
[2 marks]
HDPE is more rigid/less flexible
OR
HDPE has a higher melting point
OR
HDPE has greater «tensile» strength
Accept “HDPE has lower ductility”.
[1 mark]
form «temporary» activated complexes/reaction intermediates
Accept “consumed in one reaction/step AND regenerated in a later reaction/step”.
Accept “provides alternative mechanism”.
[1 mark]
inductively coupled plasma/ICP spectroscopy using mass spectroscopy/mass spectrometry/MS/ICP-MS
OR
inductively coupled plasma/ICP spectroscopy using optical emission spectroscopy/OES/ICP-OES
Accept “atomic absorption/aa spectroscopy” or “MS/massspectroscopy/mass spectrometry”.
[1 mark]
Any two of:
many types «of plastics» exist
OR
«plastics» require sorting «by type»
«plastics» need to be separated from non-plastic materials
OR
«often» composites/moulded on/bound to non-plastic/other components
Accept other valid factors such as thermal decomposition of some plastics, production of toxic fumes, etc.
[2 marks]
«different classifications are appropriate for» different properties/applications/purposes
[1 mark]
Question
Propene can polymerize to form polypropene.
Propene monomer: 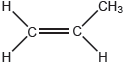
Sketch four repeating units of the polymer to show atactic and isotactic polypropene.
State the chemical reason why plastics do not degrade easily.
Compare two ways in which recycling differs from reusing plastics.
Civilizations are often characterized by the materials they use.
Suggest an advantage polymers have over materials from the iron age.
▶️Answer/Explanation
Markscheme
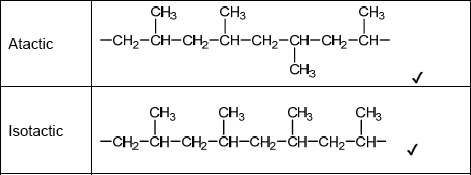
Do not accept syndiotactic (alternating orientation of the CH3 groups), eg,
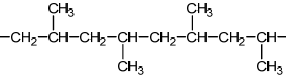
for M1 or M2.
Accept any correct atactic ordering of CH3 groups.
Penalize missing hydrogens or incorrect bond connectivities once only.
Accept skeletal structures.
Ignore continuation bonds, brackets and “n” indices in structures.
[2 marks]
strong covalent bonds
Accept “moisture cannot get inside the plastic matrix, and bacteria cannot live without moisture, so they cannot attack the polymer chains”.
Accept “bacteria lack the enzymes required to break down the hydrocarbon chains”.
[1 mark]
Any two of:
Recycling: shredded/melted/reformed AND Reuse: used in its current form
recycling is more energy intensive «than reusing»
recycling degrades the quality of plastic but reusing «typically» does not
recycling breaks down original product to form a new product whereas reuse extends product life
[2 marks]
more pliable/flexible materials
OR
more durable/non-corrosive/longer-lasting materials
OR
greater variety of materials
OR
lower density
OR
can be clear/translucent
Accept “more adaptable”.
Do not accept just “more useful”.
[1 mark]

