Question
New materials have brought many benefits to society but come with associated risks.
(a) High-pressure carbon monoxide disproportionation (HiPco) produces carbon atoms that react with nano catalysts to produce carbon nanotubes.
(i) Write the equation for the disproportionation of carbon monoxide to produce carbon atoms.
(ii) Calculate the percent atom economy of producing carbon using this method. Use section 1 of the data booklet.
(iii) Outline how a metal functions as a heterogeneous catalyst.
(iv) Explain whether the production of carbon nanotubes using HiPco is a bottom up or top down nanotechnology technique.
(v) Suggest one health risk of using nanoparticles.
(b) Kevlar® is a recyclable polyamide polymer and a liquid crystal. One repeating unit of the polyamide is shown.
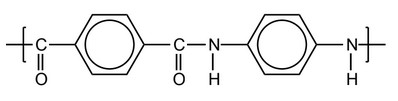
(i) Outline what is meant by a liquid crystal.
(ii) Some liquid crystal displays (LCD) use liquid crystals between two polarizing filters. The display appears black until a small voltage is applied. Outline how the liquid crystals allow polarized light to pass through the filters.
(iii) Identify the resin identification code (RIC) that applies to Kevlar®. Use section 30 of the data booklet.
The IR spectrum of Kevlar® is shown.
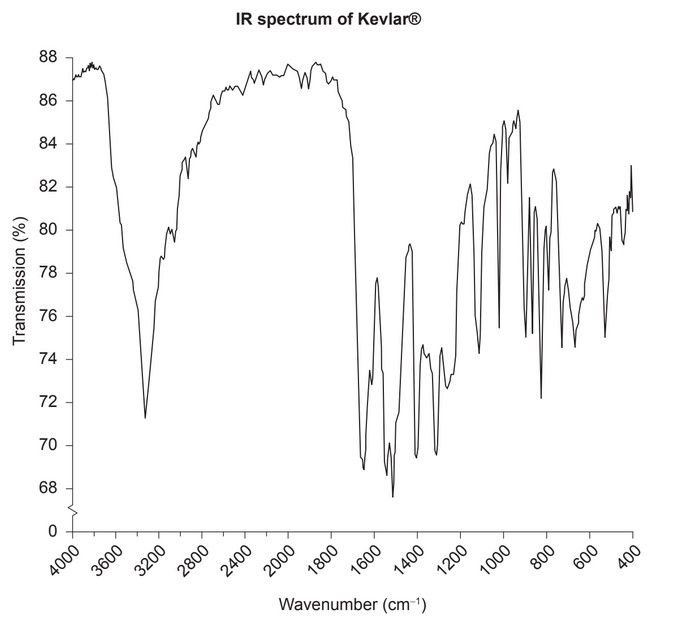
(iv) Deduce the peak in the Kevlar® IR spectrum which would not be found in compounds with any other RIC code. Use Sections 26 and 30 of the data booklet.
(v) Kevlar® is a condensation polymer. Distinguish between addition and condensation polymerization, in terms of monomers and products.
Monomers:
Products:
Answer/Explanation
Answer:
(a) \(2CO(g) \rightarrow C(s) + CO_2(g)\)
(ii) «100 × 12.01 / 2 × (12.01+16.00) = » 21.44%
(iii) «gaseous» reactants adsorb onto «metal» surface
OR
catalyst provides surface for reaction to occur
weakens «reactant» bonds
OR
products desorb
(iv) bottom up AND molecular assembly «rather than decomposition»
(v) Any one of:
more easily airborne/inhaled
have similar dimensions as biological molecules/interfere with biochemical
reactions
easily absorbed into body
may cross cell membranes
large surface area could increase toxicity
human defence system not effective with small size
(b) (i) fluids with «some» properties that are anisotropic/depend on molecular orientation «relative to a fixed axis»
(ii) polar «molecules»
change orientation upon application of electric field
OR
«in some orientations» molecules rotate plane of polarization «of polarized light»
(iii) 7
(iv) 3300 to 3500
(v) Monomers:
addition: unsaturated/containing C=C/C≡C
condensation: monomers have two reactive sites/functional groups
Products:
addition: one product/no by-products AND
condensation: small molecule/HCl eliminated/two products
Question
Carbon fibre reinforced plastic (CFRP) is a useful composite. Epoxy is a thermoset polymer that is used as a binding polymer when making CFRP.
a. Outline the two distinct phases of this composite.
b(i)Thermoplastic composites are increasingly replacing thermosets.
Suggest one advantage of thermoplastic polymers over thermosets.
b(ii )Explain how thermoplastics, such as polyvinylchloride, PVC, can be made more flexible by the addition of phthalate ester plasticizers.
b(ii)Explain why phthalates are replaced by other plasticizers in the production of plastics.
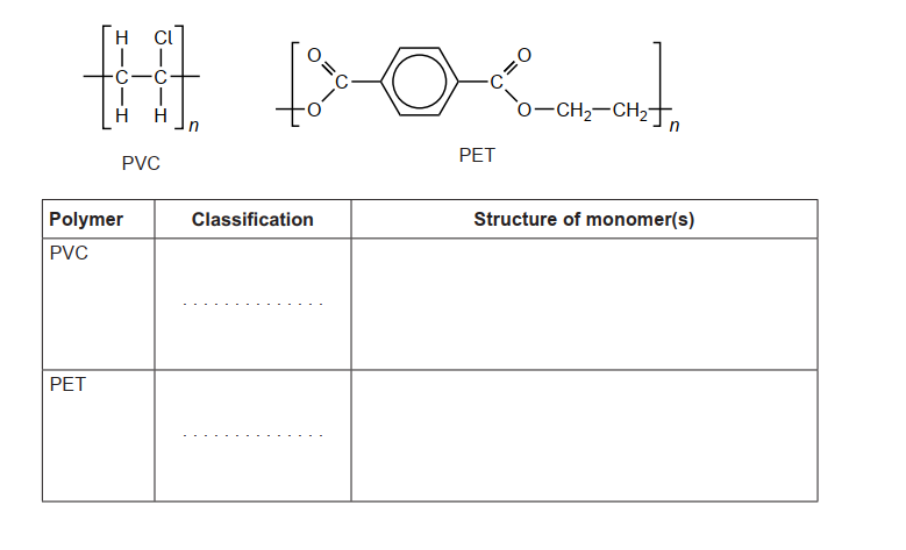
c. Classify PVC and polyethene terephthalate, PET, as addition or condensation polymers and deduce the structural formulas.
▶️Answer/Explanation
Markscheme
a. carbon fibre reinforcing phase
«in a» matrix phase of epoxy
Award [1 max] for “reinforcing phase “embedded» in a matrix”.
b(i)can be recycled
OR
can be reformed when hot
OR
high impact/chemical/abrasion resistance
b(ii)Any three of:
plasticizers embed/fit between «polymer» chains
keep polymer strands/chains/molecules separated/apart
weaken intermolecular/London/dispersion/attractive/forces/instantaneous induced dipole-induced dipole/forces «between chains» prevent chains from packing closely/forming regular packing/structure
Accept “van der Waals/vdW” for “London”.
b(iiiAny two of:
readily released into environment
OR
have weak intermolecular forces «rather than covalent bonds between chains»
get into biological systems by ingestion/inhalation
interrupt endocrine systems
OR
affect release of hormones
OR
effect development of male reproductive system
considered carcinogenic
OR
can cause cellular damage
can cause early puberty in females
can cause thyroid effects
can cause asthma
Do not accept just “are a health concern”.
c.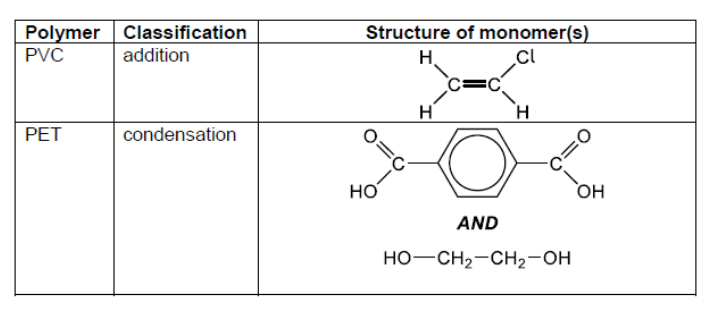
PVC: addition AND PET: condensation structure of PVC monomer structure of PET monomers Accept full OR condensed structural formulas.
Question
Polybutadiene, used in truck tyres, is a polymer of buta-1,3-diene. The spatial arrangement of atoms in the polymer depends on the type of catalyst used.
a. Outline two differences between heterogeneous and homogeneous catalysts.
b. Suggest, giving a reason, how elastomers used for the tyre tread can increase the traction between the tyre and the road.
c. Tyre fires emit trace quantities of polychlorinated dibenzofurans and polychlorinated dibenzo-p-dioxin.
Outline, using section 31 of the data booklet, why polychlorinated dibenzofuran is not classed chemically as a dioxin but considered “dioxin-like”.
d. Classify polybutadiene as either an addition or condensation polymer, giving a reason.
e. State one factor considered when making green chemistry polymers.
▶️Answer/Explanation
Markscheme
a. Any two of:
heterogeneous catalyst is in different phase than reactants AND homogeneous catalyst in same phase [ $U$ ]
homogeneous catalysts chemically change/react and reformed at end of reaction
OR
reactants adsorb onto heterogenous catalyst and products desorb [ $\quad[]$
heterogeneous catalysts are more easily removed than homogenous catalysts $[\boldsymbol{V}]$
heterogeneous catalysts can function at higher temperatures
[ $\checkmark]$
homogeneous catalysts are «generally» more selective
[
homogeneous catalysts offer a broader range of reactions
[v]
Note: Accept “state” for “phase”.
Accept “heterogeneous catalyst provides a surface to activate reaction”.
b. elastomers bend under force «and return to original form when force is released»
OR
elastomers make tyre more flexible [ $\boldsymbol{]}$
allows greater contact with road $[\boldsymbol{M}$
c. does not contain heterocyclic ring with 2 oxygen atoms
OR
middle ring has only 1 oxygen atom [ $[\boldsymbol{C}$
produces similar toxic effects to dioxins [ $\boldsymbol{V}]$
d. addition $A N D$ not two different functional groups reacting
OR
addition AND formed by breaking one bond of the carbon-carbon double bonds
OR
addition $\boldsymbol{A N D}$ empirical formula of monomer equals empirical formula of polymer
OR
addition $\boldsymbol{A N D}$ no atoms removed/all atoms accounted for/no loss of water/ammonia/inorganic by-product/small molecules
OR
addition $\boldsymbol{A N D}$ atom economy/efficiency is $100 \%$
OR
addition $\boldsymbol{A N D}$ there is only one «reaction» product [ $\boldsymbol{U}$ ]
e. Any one of:
high content of raw materials in product/high atom economy [ [ ]
use of low toxic chemicals/catalysts/materials/solvents $\quad[\boldsymbol{\nu}]$
renewable feedstock/raw materials
use of renewable/clean/low carbon energy source $[\boldsymbol{\sim}]$
high safety standards
[ $\boldsymbol{\sim}]$
increase energy efficiency
$[\boldsymbol{v}]$
waste recycling $[\boldsymbol{\sim}]$
Note: Accept other reasonable answers.
