Question
a(i)Proteins are polymers of amino acids.
A paper chromatogram of two amino acids, A1 and A2, is obtained using a non-polar solvent.
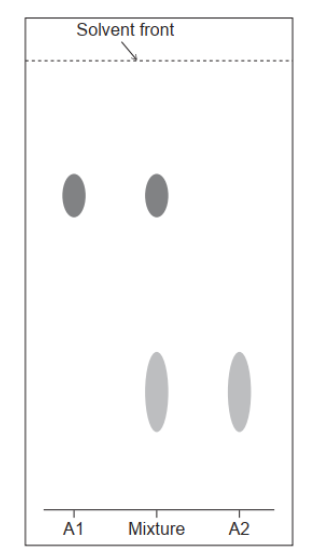
Determine the $R_f$ value of $A 1$.
a(ii).roteins are polymers of amino acids.
The mixture is composed of glycine, Gly, and isoleucine, Ile. Their structures can be found in section 33 of the data booklet. Deduce, referring to relative affinities and $R_f$, the identity of $A 1$.
b. Proteins are polymers of amino acids.
Glycine is one of the amino acids in the primary structure of hemoglobin.
State the type of bonding responsible for the $\alpha$-helix in the secondary structure.
c(i)Proteins are polymers of amino acids.
Sketch and label two oxygen dissociation curves, one for adult hemoglobin and one for foetal hemoglobin.
c(ii).roteins are polymers of amino acids.
Explain why the affinity for oxygen of foetal hemoglobin differs from that of adult hemoglobin.
▶️Answer/Explanation
Markscheme
$\mathrm{a}(\mathrm{i}) 0.70$
Accept any value within the range “0.67-0.73”.
a(ii)le $\boldsymbol{A N D}$ larger $\mathrm{R}_{\mathrm{f}}$
more soluble in non-polar solvent «mobile phase»
OR
not as attracted to polar «stationary» phase
Only award M2 if lle is identified in M1.
b. hydrogen/H bonding «between amido hydrogen and carboxyl oxygen atoms»
c(i).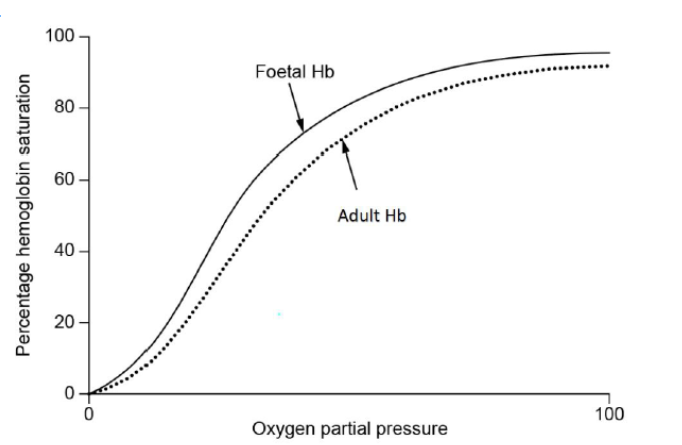
both curves sigmoidal shape AND starting at zero
foetal hemoglobin showing greater affinity/steeper/higher gradient
Do not penalize if convergence is not approached for $M 1$.
Both curves must be labelled to score M2.
c(ii)Any two of:
contains two gamma/ $\gamma$ units «instead of two beta/ $\beta$ units found in adults» OR
differs in amino acid sequence «from the two beta// $\beta$ units found in adults» less sensitive to inhibitors/2,3-BPG/DPG
receives $\mathrm{O}_2$ from «partly deoxygenated» blood so can work at low $\mathrm{pO}_2$
low $\mathrm{p} \mathrm{CO}_2$ in foetal blood increases affinity for $\mathrm{O}_2$
hemoglobin concentration in foetal blood greater than in the mother
Question
Proteins have structural or enzyme functions.
Oil spills are a major environmental problem.
a(i)Some proteins form an a-helix. State the name of another secondary protein structure.
a(iiCompare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
b. Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
c(i)Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
c(ii) Oil spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
▶️Answer/Explanation
Markscheme
a(i) $\beta /$ beta pleated/sheet $[\boldsymbol{\sim}]$
a(ii) One similarity:
hydrogen bonding
OR
attractions between $\mathrm{C}=\mathrm{O}$ and $\mathrm{N}-\mathrm{H}[\boldsymbol{W}]$
One difference:
$\alpha$-helix has hydrogen bonds between amino acid residues that are closer than $\beta$-pleated sheet
OR
H-bonds in a-helix parallel to helix axis AND perpendicular to sheet in $\beta$-pleated sheet
OR
a-helix has one strand $A N D \beta$-pleated sheet has two «or more» strands
OR
a-helix is more elastic «since $\mathrm{H}$-bonds can be broken easily» AND $\beta$-pleated sheet is less elastic «since H-bonds are difficult to break» [ $\sim$ ]
Note: Accept a diagram which shows hydrogen bonding between $\mathrm{O}$ of $\mathrm{C}=\mathrm{O}$ and $\mathrm{H}$ of $\mathrm{NH}$ groups for $\mathrm{M1}$.
Accept “between carbonyl/amido/amide/carboxamide” but not “between amino/amine” for M1.
b. enzyme denatured/loss of 3-D structure/conformational change
OR
«interactions responsible for» tertiary/quaternary structures altered [ $\boldsymbol{]}]$
shape of active site changes
OR
fewer substrate molecules fit into active sites [ $\boldsymbol{V}]$
c(i) Any two of:
surface water is warmer «so faster reaction rate»/more light/energy from the sun $[\boldsymbol{V}]$
more oxygen «for aerobic bacteria/oxidation of oil» $[\boldsymbol{\checkmark}]$
greater surface area $[\boldsymbol{\sim}]$
c(ii)Any one of:
non-hazardous/toxic to the environment/living organisms $[\boldsymbol{U}]$
energy requirements «during production» $[\boldsymbol{C}]$
quantity/type of waste produced «during production»
OR
atom economy [ $\boldsymbol{W}]$
Note: Accept “use of solvents/toxic materials “during production»”.
Do not accept “more steps involved”.
Question
Aspartame is formed from the two amino acids aspartic acid (Asp) and phenylalanine (Phe).
Chromatography is used in the analysis of proteins in the food and pharmaceutical industry.
a. Draw the structure of the dipeptide Asp-Phe using section 33 of the data booklet.
b(i)Describe, using another method, how a mixture of four amino acids, alanine, arginine, glutamic acid and glycine, could be separated when placed in a buffer solution of $\mathrm{pH} 6.0$.
b(iißSuggest why alanine and glycine separate slightly at $\mathrm{pH} 6.5$.
$\mathrm{b}$ (iiifalculate the ratio of $\left[\mathrm{A}^{-}\right]:[\mathrm{HA}]$ in a buffer of $\mathrm{pH} 6.0$ given that $\mathrm{p} K_{\mathrm{a}}$ for the acid is 4.83 , using section 1 of the data booklet.
▶️Answer/Explanation
Markscheme
a.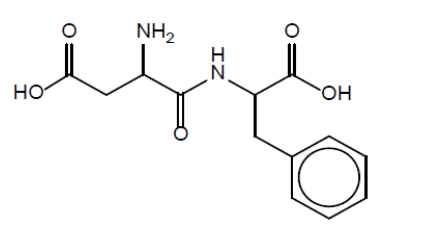
amide link (eg, CONH)
correct order and structures of amino acids
NOTE: Accept a skeletal formula or a full or condensed structural formula.
Accept zwitterion form of dipeptide.
Accept CO-NH but not CO-HN for amide link.
b(i)Any three of:
«gel» electrophoresis «technique»
OR
mixture «in buffer solution» placed on gel/paper
voltage/potential «difference» applied
amino acids move differently «depending on $\mathrm{pH} /$ isoelectric point»
compare/measure distances travelled $R_{\mathrm{f}}$ values
NOTE: Accept “mixture placed on plate covered with polyacrylamide “gel» OR “mixture put in a gel “medium»”.
b(ii) ifferent sizes/molar masses/chain lengths «so move with different speeds»
NOTE: Do not accept “different side-chains/R-groups/number of carbons”.
\begin{aligned}
& \mathrm{b}(\mathrm{iii}) 6.0=4.83+\log \frac{\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]} » \\
& \text { «log } \frac{\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}=1.17 » \\
& \text { «[ } \left.\mathrm{A}^{-}\right]:[\mathrm{HA}]=» 14.8: 1 \\
& \text { NOTE: Accept “15:1”. } \\
& \text { Do not accept 1:14.8. }
\end{aligned}
Question
a. The graph shows the relationship between the temperature and the rate of an enzyme-catalysed reaction.
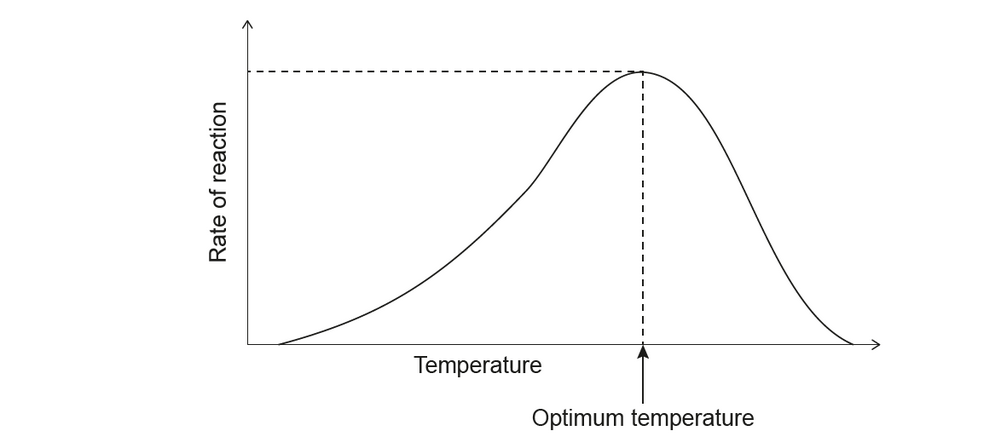
b. Explain why a change in $\mathrm{pH}$ affects the tertiary structure of an enzyme in solution.
c. State one use of enzymes in reducing environmental problems.
▶️Answer/Explanation
Markscheme
a. enzyme denatures
OR
change of conformation/shape of active site
OR
substrate cannot bind to active site/binds less efficiently
NOTE: Accept “change in structure” or “substrate doesn’t fit/fits poorly into active site”
acidic/basic/ionizable/ $\mathrm{COOH} /$ carboxyl/ $\mathrm{NH}_2 /$ amino groups in the $\mathrm{R}$ groups/side chains «react»
exchange/lose/gain protons/ $\mathrm{H}^{+}$
change in H-bonds/ionic interactions/intermolecular forces/London dispersion forces
NOTE: Do not accept “enzyme denatures” OR “change of conformation/tertiary structure” OR “substrate cannot bind to active site/binds less efficiently” as this was the answer to 8(a).
c. breakdown of oil spills/industrial/sewage waste/plastics
OR
production of alternate sources of energy «such as bio diesel»
OR
involve less toxic chemical pathway «in industry»
NOTE: Accept “«enzymes in” biological detergents can improve energy efficiency”.
Question
Myoglobin is a globular protein found in the muscle tissue and is formed from 2-amino acids.
Describe the characteristic properties of 2-amino acids.
State the name of the bond or interaction that is responsible for linking the amino acids together in the primary structure.
State the name of the bond or interaction that is responsible for the secondary structure.
State two of the bonds or interactions responsible for the 3D shape of myoglobin.
▶️Answer/Explanation
Markscheme
isoelectric point;
zwitterion/forms neutral ion when \({{\text{H}}^ + }\) transfers from COOH to \({\text{N}}{{\text{H}}_{\text{2}}}\);
buffer action/can accept \({{\text{H}}^ + }\) and OH–;
general formula \({\text{HOOCCRHN}}{{\text{H}}_{\text{2}}}\);
peptide/amide;
hydrogen bonds;
hydrogen bonds;
disulfide bridges/bonds;
ionic interactions/bonds;
van der Waals’ forces / hydrophobic interactions / London/dispersion forces / temporary induced dipoles;
Examiners report
Apart from the general formula in (a), many candidates had difficulty providing the characteristic properties of 2 – amino acids. The characteristic properties are clearly identified in the syllabus details.
Most candidates could name the correct bond types in part (b).
Most candidates could name the correct bond types in part (b).
Most candidates could name the correct bond types in part (b).
Question
The following diagram represents a thin-layer chromatogram of an amino acid.
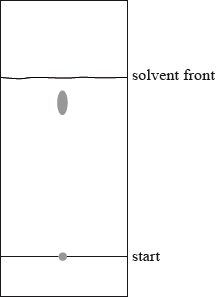
Calculate the \({R_{\text{f}}}\) of the amino acid.
▶️Answer/Explanation
Markscheme
\({R_{\text{f}}} = \frac{{40{\text{ (mm)}}}}{{46{\text{ (mm)}}}} = 0.87\);
Allow 0.86 to 0.88.
No mark if \({R_{\text{f}}}\) has units.
Examiners report
There were difficulties in the calculation of \({R_{\text{f}}}\), some candidates did not seem to know what it means, others what to measure.
Question
Proteins are vital components of living systems.
State the general formula of 2-amino acids.
State two characteristic properties of 2-amino acids.
Using Table 19 of the Data Booklet, deduce the structural formula of two dipeptides that could be formed by the reaction of alanine with serine and state the other product of the reaction.
Other product of the reaction:
Explain the difference between the primary and secondary structure of proteins.
State the predominant interaction responsible for the secondary structure.
Explain how a sample of a protein can be analysed by electrophoresis.
▶️Answer/Explanation
Markscheme
H2NCHRCOOH;
Allow various other combinations e.g. RCH(NH2)COOH etc. and allow NH2 and HOOC on right etc.
Allow structural formula if drawn, showing all the bonds.
Do not accept the formula of a specific amino acid.
isoelectric point;
formation of zwitterion/inner salt / \({{\text{H}}_{\text{3}}}{{\text{N}}^ + }{\text{CHRCO}}{{\text{O}}^ – }\);
(can act as a) buffer / has both acidic and basic properties / can react with \({{\text{H}}^ + }\) or \({\text{O}}{{\text{H}}^ – }\) / can exist as cations in acidic solution and anions in alkaline solution;
can form proteins/dipeptides/peptides / can react to form condensation products;
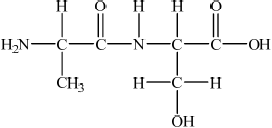 ;
;
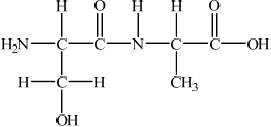 ;
;
Allow condensed structural formulas.
water/ H2O;
Primary structure:
(linear) sequence/order of amino acids / OWTTE;
Secondary structure:
way in which chain of amino acids folds itself / way in which sequence is kept together by hydrogen bonding between atoms in sequence / OWTTE;
Accept can exist as \(\alpha \)-helix or \(\beta \)-sheet.
hydrogen bonding/ H- bonding;
add hydrochloric acid/HCl / hydrolyse to convert protein into amino acid mixture / (successively) release amino acids;
mixture/amino acids spotted/placed on paper/gel;
Can be shown with diagram.
Do not accept protein placed/spotted on paper/gel.
use of buffer solution;
apply voltage/potential difference;
Can be shown with diagram.
Do not allow “pass current/electricity through mixture”.
amino acids move in different directions (depending on their isoelectric points);
develop with ninhydrin/triketohydrindane hydrate/2,2-dihydroxyindane-1,3-dione/organic dye;
measure distances moved / compare with known samples / measure isoelectric points (and compare with data);
Examiners report
Most attempts at (a) were successful.
A surprising number of candidates failed in (b) to give properties, as they misinterpreted the question and quoted structural features, such as the presence of an amino group.
Some students had difficulty deducing the structure of the two dipeptides in (c), however most candidates were able to identify the other product as water.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Most answers in (d) showed an understanding of protein primary and secondary structures.
Responses to (e) were better than in some previous sessions; even so, full marks were rare, with many either omitting the hydrolysis of the protein or referring to a current being passed through the sample rather than a voltage being applied.
Question
Individual 2-amino acids have different structures depending on the pH of the solution they are dissolved in. The structures of serine and cysteine are given in Table 19 of the Data Booklet.
Deduce the structure of serine in
(i) a solution with a pH of 2.
(ii) a solution with a pH of 12.
Deduce the structure of serine at the isoelectric point.
Deduce the structures of the two different dipeptides that can be formed when one molecule of serine reacts with one molecule of cysteine.
▶️Answer/Explanation
Markscheme
(i) 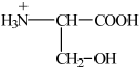 ;
;
If R– used or incorrect amino acid structure chosen from data book apply ECF for subsequent answers.
(ii) 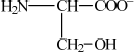 ;
;
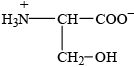 ;
;
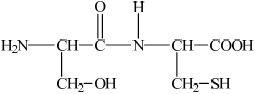 ;
;
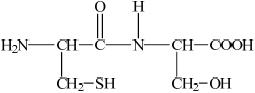 ;
;
Accept –CO–NH–/–CO–HN– for peptide linkage.
Examiners report
Most candidates simply drew the structure of the amino acid from the Data Booklet, and did not indicate the conjugate acid or base of the amino acid in solution in part (a).
Few knew how to draw the structure of the zwitterion in part (b). One G2 respondent commented that deducing the structure of an amino acid at varying pH levels is not on the syllabus. It is, in fact, referred to in B.2.2.
The better candidates were able to draw structures of two dipeptides.
Many weaker candidates were unable to create peptide links, and joined the molecules creatively but incorrectly.
Question
Paper chromatography may be used to separate a mixture of sugars.
(a) State the stationary phase and an example of a mobile phase used in paper chromatography.
Stationary phase:
Mobile phase:
(b) The identity of two sugars in a mixture can be determined by measuring their \({R_{\text{f}}}\) values, after staining.
(i) Describe how an \({R_{\text{f}}}\) value can be calculated.
(ii) Calculate the \({R_{\text{f}}}\) value for sugar 2 in the chromatogram below.
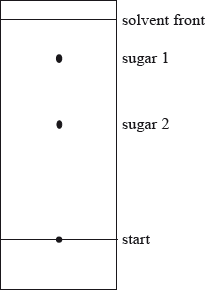
(c) Explain how the \({R_{\text{f}}}\) value of sugar 2 could be used to identify it.
▶️Answer/Explanation
Markscheme
(a) Stationary phase:
water in the paper;
Mobile phase:
water / any other non corrosive solvent or solvent mixture;
(b) (i) \({R_{\text{f}}} = \frac{{{\text{distance moved by substance}}}}{{{\text{distance moved by solvent / solvent front}}}}\);
(ii) \({R_{\text{f}}} = \frac{{3.0}}{{5.7}} = 0.53\);
Accept answers between 0.5 and 0.55 (± 1mm on measurements).
(c) compare \({R_{\text{f}}}\) of unknown to known values;
(conducted) under the same conditions (of stationary/mobile phase);
Allow second mark for specifying a particular condition to keep the same (e.g. solvent).
Examiners report
This was generally well done with most candidates displaying a good comprehension of the \({R_{\text{f}}}\) concept. In Part (c) many candidates correctly suggested comparing the \({R_{\text{f}}}\) value to that of standard samples, but very few pointed out that these must be obtained under identical conditions.
Question
Proteins are natural polymers.
(a) List four major functions of proteins in the human body.
(b) Deduce the structures of two different tripeptides that can be formed when all three amino acids given below react together.

(c) Deduce the number of tripeptides that could be formed by using all three of the above amino acids to form a tripeptide.
(d) State the type of bonding that is responsible for the primary and secondary structures of proteins.
Primary:
Secondary:
(e) Describe and explain the tertiary structure of proteins. Include in your answer all the bonds and interactions responsible for the tertiary structure.
▶️Answer/Explanation
Markscheme
(a) structure / growth / repair
enzymes
hormones
transport
immunoproteins/antibodies
energy source
Two functions score [1].
(b) 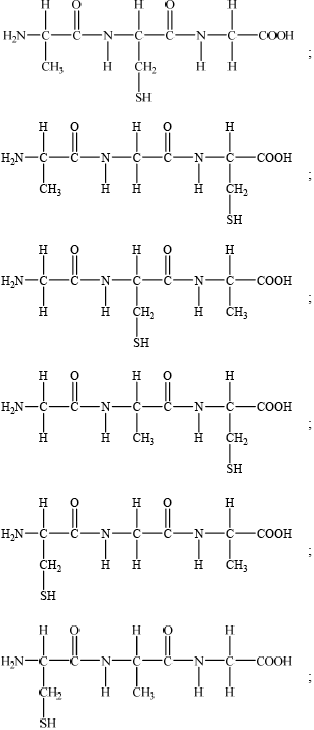
Accept CONH for peptide bond.
Penalize incorrect representation of peptide bond (e.g. COHN) once only.
(c) 6;
(d) 
(e) secondary structure folds to form a (unique) 3-D/dimensional structure of the protein;
Structure stabilized by:
covalent bonds / disulfide bridges
hydrogen/H-bonding
ionic bonds / salt bridges
van der Waals’/dispersion/London forces
Two bond types score [1].
Examiners report
Often quite well done. In part (a), whilst proteins can be used as an energy source, energy storage would not be considered a major function as the body usually stores energy in other forms. In Part (e), few candidates pointed out that the tertiary structure is a folding of the primary and secondary structures that gives the protein its three-dimensional shape.
Question
A mixture of the amino acids serine (Ser), glutamic acid (Glu) and lysine (Lys) was separated using electrophoresis and a buffer of pH 5.7. A drop containing the mixture was placed in the centre of the paper and a potential difference was applied. The amino acids were developed and the following results were obtained.
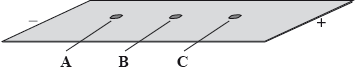
Describe how the amino acid spots may have been developed.
Predict which amino acid is present at spot C. Explain your answer.
The amino acid at spot B is at its isoelectric point. Describe one characteristic of an amino acid at its isoelectric point.
Explain, using equations, how the amino acid glycine (Gly) can act as a buffer
▶️Answer/Explanation
Markscheme
organic dye / ninhydrin;
glutamic acid/Glu;
isoelectric point is below pH of buffer / acts as an acid / loses \({{\text{H}}^ + }\);
becomes negatively charged;
balanced (positive and negative) charges / no overall charge / zwitterion;
amphoteric / buffer solution;
\({{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{COOH}} + {{\text{H}}^{\text{ + }}} \rightleftharpoons {{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{COOH}}/{{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ – } + {{\text{H}}^ + } \rightleftharpoons {{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{COOH}}\);
\({{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{COOH}} + {\text{O}}{{\text{H}}^ – } \rightleftharpoons {{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ – } + {{\text{H}}_2}{\text{O}}/\)
\({{\text{H}}_3}{{\text{N}}^ + }{\text{C}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ – } + {\text{O}}{{\text{H}}^ – } \rightleftharpoons {{\text{H}}_2}{\text{NC}}{{\text{H}}_2}{\text{CO}}{{\text{O}}^ – } + {{\text{H}}_2}{\text{O}}\)
Accept \( \to \) instead of \( \rightleftharpoons \)
Examiners report
Many candidates described electrophoresis instead of stating that ninhydrin was used to develop the amino acid spots. The process of electrophoresis was detailed in the stem of the question, so candidates should have been able to determine what was required if the question had been read carefully.
Predicting which amino acid was closer to the positive electrode was challenging, although many candidates scored some marks for their reasoning.
The majority of candidates correctly described one characteristic of an amino acid at its isoelectric point.
In (b) very few could write equations to explain how glycine can act as a buffer. Most candidates answered in words only, even though equations were specifically requested. A G2 comment suggested that SL candidates did not need to know about buffers. This is clearly stated as a requirement in B.2.2.
Question
Proteins are macromolecules formed from 2-amino acids. Once a protein has been hydrolysed, chromatography and electrophoresis can be used to identify the amino acids present.
State the name of the linkage that is broken during the hydrolysis of a protein and draw its structure.
Explain how electrophoresis is used to analyse a protein.
▶️Answer/Explanation
Markscheme
peptide/amide;
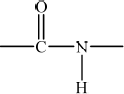 ;
;
Continuation bonds are needed for the mark.
add HCl/NaOH/enzyme (to hydrolyse the protein into amino acids);
mixture of amino acids is placed on the centre of a gel/PAGE/polyacrylamide/paper in buffer solution;
voltage/potential difference applied across gel;
Do not accept electric current.
different amino acids move to different distances according to their charge/isoelectric point / move at different rates towards oppositely charged electrodes;
gel/paper developed by spraying with ninhydrin/organic dye/can be detected by a stain/made to fluoresce under ultra-violet light;
distances moved/isoelectric points are compared with literature values;
Examiners report
It was surprising to see that quite a few candidates did not know the name of the linkage broken during the hydrolysis of a protein and only about half of the candidates, who stated the name could draw the structure of the peptide bond correctly. In some cases glycosidic or ester linkage appeared.
The explanation of how electrophoresis is used to analyse a protein was generally answered very well.
Question
Most foods are complex mixtures and many components of them are nutrients.
Identify the types of nutrients A, B and C.
A 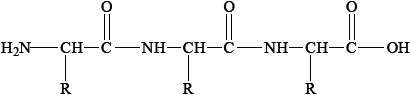
B 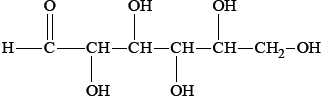
C 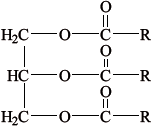
State the names of two types of nutrient other than those shown in part (b).
▶️Answer/Explanation
Markscheme
A: protein / polypeptide/tripeptide;
B: carbohydrate / sugar / monosaccharide / glucose;
C: lipid / triglyceride / vegetable oil / fat;
vitamins;
minerals;
water;
Examiners report
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
This question was generally well answered by many candidates. Though most had a general idea of the difference between a food and a nutrient, many did not appreciate the distinction between an “unhealthy” food and one that isn’t a nutrient.
Question
Proteins are formed during condensation reactions of 2-amino acids.
Using Table 19 of the Data Booklet, deduce the structural formulas of the two dipeptides formed by the reaction of leucine (Leu) with valine (Val).
Dipeptide 1:
Dipeptide 2:
State the other substance formed during this reaction.
Explain how amino acids can be analysed using electrophoresis.
List two functions of proteins in the body.
▶️Answer/Explanation
Markscheme
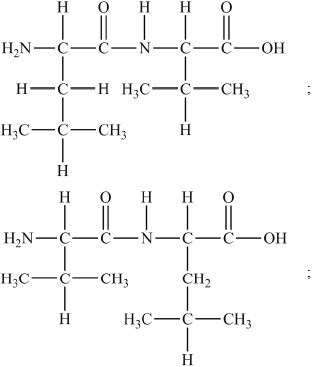
Accept full or condensed structural formulas.
Penalize incorrect representation of peptide link (COHN or NHOC) once.
Award [1] for a correct peptide link if the rest of the structure is incorrect.
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
sample of amino acids/mixture placed/spotted on gel/polyacrylamide/PAGE/paper and buffer solution/solution of known pH;
potential difference/voltage applied;
Do not accept current.
Allow potential for potential difference.
Allow electric field applied.
if the (amino acid’s) isoelectric point is below the pH (of buffer) it is negatively charged / if the (amino acid’s) isoelectric point is above the pH (of buffer) it is positively charged;
different amino acids move different distances/rates according to their charge/isoelectric point / different amino acids move at different rates towards oppositely charged electrodes / OWTTE;
spray/develop with ninhydrin/organic dye / detected by staining/fluorescence under UV light;
measure distance travelled and compare with standards/isoelectric points;
Any two for [1]
structural / growth / repair
Allow more specific function eg, forms tendons/muscles/eye lens/ nails/hair, repair of tissue/cells etc.
enzyme / biological catalyst
hormone / chemical messenger
transport of molecules
Allow movement/carriage of molecules, and chemicals instead of molecules / OWTTE.
Do not award mark for transport alone.
storage of molecules
Do not award mark for storage alone.
Allow chemicals instead of molecules / OWTTE.
lubrication
(to make/produce) immunoproteins/antibodies
energy source
Do not accept energy storage.
Allow more specific examples of any of the above.
Examiners report
Part (a) was generally well answered. There were instances of careless mistakes where the side-chain of the amino acid was incorrectly copied or connected, and some instances where peptide links were incorrectly represented as COHN resulting in the loss of one mark. However, there were many cases where candidates had totally incorrect links between the amino acids in the dipeptide, and some scripts did not even attempt to connect the two amino acids.
Part (a) was generally well answered. There were instances of careless mistakes where the side-chain of the amino acid was incorrectly copied or connected, and some instances where peptide links were incorrectly represented as COHN resulting in the loss of one mark. However, there were many cases where candidates had totally incorrect links between the amino acids in the dipeptide, and some scripts did not even attempt to connect the two amino acids.
In part (b), all candidates were reasonably familiar with describing electrophoresis and some of them tried to explain how electrophoresis separated amino acids, but most candidates did not show a thorough understanding of the technique and only scored partial marks out of the 4 marks allocated. Most candidates did not clarify that amino acids moved at different rates through the gel depending on their charge (at the buffer’s pH), or the relation between isoelectric point, buffer pH and charge on amino acid.
The majority of candidates stated two correct functions of proteins in part (c). Energy storage was not accepted, and transport needed mention of molecules.
Question
Paper chromatography is a simple method used to separate and identify the components in a mixture. To aid identification, the retention factor, \({R_{\text{f}}}\), of an unknown component can be compared with the \({R_{\text{f}}}\) values of pure samples of the possible components.
State the meaning of the term retention factor.
Explain why the value of the retention factor for the same component can be very different if different solvents (eluents) are used for the mobile phase.
If the components of the mixture are coloured then they can be seen with the naked eye. Describe two different ways in which a chromatogram can be developed if the components are colourless.
▶️Answer/Explanation
Markscheme
The ratio between the distance moved by the spot and the distance moved by the solvent front / OWTTE;
Accept this expressed as a correct equation.
\({R_{\text{f}}}\) value depends on the intermolecular forces that the component has with the mobile phase compared to the stationary phase / relative attraction of the component to mobile phase compared to the stationary phase / partition of the component between the mobile phase and the stationary phase / OWTTE;
if polarity of the solvents is different the intermolecular forces/attraction to mobile phase/partition will be different / OWTTE;
Accept “Components have different solubilities in different solvents” / OWTTE.
(viewing under) ultraviolet/UV light;
(staining with) a dye/ninhydrin/potassium manganate(VII);
(exposing to) iodine (vapour);
Accept “staining with a developing (re)agent”.
Do not accept just staining.
Examiners report
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
Many students could correctly define “retention factor” though they frequently used the term “solute” rather than “component” and “solvent” rather than “eluent front”. Very few candidates were capable of describing, in sufficient detail to gain the second mark, how the properties of the eluent, such as its polarity or ability to engage in particular types of intermolecular bonding, affected the relative attraction of a particular component for the stationary and mobile phases, and hence the distance it would travel relative to the eluent front; indeed many said that as the distance travelled by the eluent might vary so the Rf value would vary. About half the candidates were aware of some technique to identify colourless components.
Question
Papain is a globular protein which is present in papaya fruit. Part of the sequence of its polypeptide chain is Gly–Cys–Val–Gly.
In the analysis of proteins, mixtures of amino acids with different isoelectric points can be separated using electrophoresis.
Proteins such as papain are formed by the condensation reactions of 2-amino acids.
By referring to Table 19 of the Data Booklet, draw the structural formulas of the two dipeptides formed by the reaction of glycine with cysteine.
Describe the essential features of electrophoresis.
Arginine, cysteine and glycine undergo electrophoresis at pH 6.0. Deduce which amino acid moves towards the positive electrode (anode).
Describe what is meant by the tertiary structure of proteins.
Identify two interactions which are responsible for this type of structure.
▶️Answer/Explanation
Markscheme
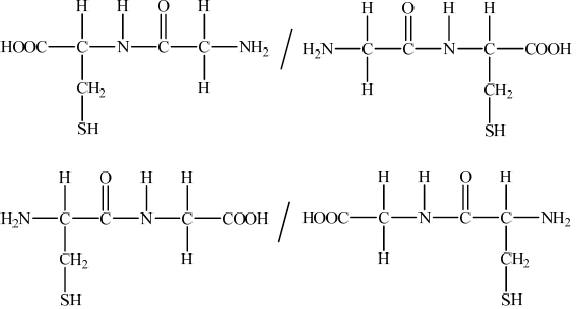
Accept condensed versions of structures such as –NH–CO–CH2– / –HN–CO–CH2–
Penalise repeated minor errors, such as incorrect representation of peptide bond (–COHN–/–NHOC–) once only.
Award [1] for a correct peptide link if the rest of the structure is incorrect.
sample of amino acids/mixture placed/spotted on gel/ polyacrylamide/PAGE/paper;
buffer solution / solution of known pH;
(high) potential (difference)/electric field/voltage applied / + and – electrodes/anode and cathode connected;
Accept current/electricity passed through.
different amino acids move different rates/distances according to their charge/isoelectric point / amino acids move towards oppositely charged electrode / OWTTE;
spray/develop with ninhydrin/organic dye/ detect by staining/fluorescence under UV light;
measure distance travelled and compare with standards/isoelectric points;
Award [1 max] for the statement “different amino-acids move to different extents”.
cysteine;
folding of secondary structure / produces 3D shape of the protein;
Do not accept “folding of the protein chain” / OWTTE.
hydrogen/H bonds
ionic bonds/attraction
van der Waals’/London/dispersion forces
disulfide bridges
hydrophobic interactions
Award [1] for any two of the above.
Examiners report
Whilst some candidates were unaware of how amino acids might join (or even what they were!) many could correctly write the structures of the two possible dipeptides. Many candidates knew the basics of electrophoresis, but the high scores they achieved were also a reflection of a generous markscheme and this lack of a sound understanding was often reflected in their inability to correctly identify the acid that would move to the anode. Whilst many candidates knew interactions responsible for the tertiary structure of proteins, their descriptions of what this was often failed to differentiate it from secondary structure.c.ii.
Question
Proteins are polymers of 2-amino acids. The structures of the common amino acids are given in Table 19 of the Data Booklet. This question refers to the two amino acids alanine and cysteine.
With reference to the isoelectric points of alanine and cysteine:
State the structural formula of cysteine as a zwitterion.
identify a pH value where both amino acids would be positively charged.
describe with a reason what pH value would be suitable to use in an electrophoresis experiment designed to separate these two amino acids in solution.
Cysteine is responsible for a specific type of intra-molecular bonding within a protein molecule. State the name of this type of interaction and outline how it is different from other interactions responsible for the tertiary structure.
State three functions of proteins in the body and include a named example for each.
▶️Answer/Explanation
Markscheme
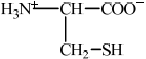 ;
;
Accept full or condensed structural formulas as long as correct charges on N/NH3 and O are represented.
Accept NH3+ for H3N+ in the diagram.
any value or range below 5.1;
any value or range from 5.1-6.0;
alanine positive and cysteine negative;
Accept biggest charge difference/opposite charges between isoelectric points so move in opposite directions.
Need reference to charges to score M2.
disulfide bridge;
Accept S–S.
covalent / strongest bond;
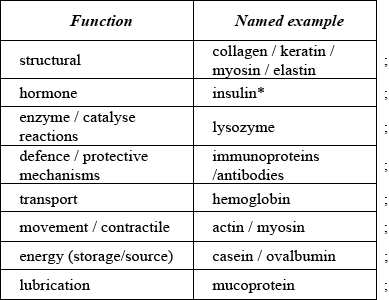
Need function with valid example for each mark.
Award [1 max] for three correct functions or three named examples.
Accept other correct examples.
Do not apply list principle.
* Other protein hormones include human growth hormone, follicle stimulating hormone (FSH), adrenocorticotropic hormone (corticotropin or ACTH), thyroid stimulating hormone (TSH).
* Do not accept steroids, sex hormones, testosterone, progesterone, estrogen, adrenalin.
Examiners report
The zwitterion mark was often lost (missing H or charge on the amine end) but pH values in (b) were correctly identified. The second mark in (b)(ii) was often lost because there was no reference to charges and the direction of migration. About half of the candidates correctly identified the disulfide bridge but fewer were able to outline the difference from other tertiary structure interactions. Part (d) caused problems for all but well-prepared candidates; they need a good grasp of subject-specific vocabulary
Question
A sample is known to contain three different amino acids. After carrying out paper chromatography using a solvent made up of propan-1-ol, water and ammonia, the following chromatogram was obtained once the spots had been developed with ninhydrin.
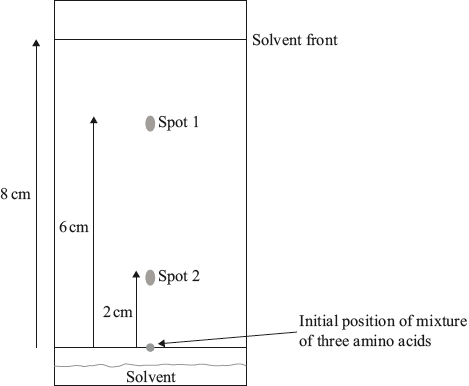
Calculate the \({R_{\text{f}}}\) values for the two spots.
Spot 1:
Spot 2:
Suggest a reason why only two spots are present.
Suggest how the chromatography experiment with the same sample could be altered in order to obtain three spots.
▶️Answer/Explanation
Markscheme
Spot 1: \({R_{\text{f}}} = 0.75\) and Spot 2: \({R_{\text{f}}} = 0.25\);
two amino acids have the same \({R_{\text{f}}}\) value;
change the polarity/make-up of the solvent / use a different solvent;
Accept two way (paper) chromatography.
Examiners report
This question was generally well answered. In part (a) some candidates reversed the answers for the spots and lost the mark. Very few linked the \({R_{\text{f}}}\) value in part (b) to the two amino acids on same spot; often the reason stated was that one amino acid did not dissolve. Part (c) was well answered. Students demonstrated a good understanding of how the nature of the solvent affects the \({R_{\text{f}}}\) value.
Question
Thin-layer chromatography (TLC) is an example of adsorption chromatography.
A mixture of two organic compounds was separated by TLC using a non-polar solvent.
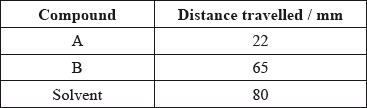
(i) Calculate the \({R_{\text{f}}}\) values of A and B.
(ii) Outline why compound B has travelled the greater distance.
▶️Answer/Explanation
Markscheme
(i) 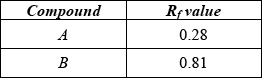 ;
;
Award [1] for both correct.
(ii) B is more soluble in solvent/mobile phase / B is less polar than A / B is less strongly adsorbed onto stationary phase;
Accept B is non-polar.
Do not allow “greater attraction/affinity to solvent” without reference to solubility.
Examiners report
(i) Very well answered. Many candidates used an inappropriate number of significant figures for the calculated \({R_{\text{f}}}\) values, but this was not penalized in this instance.
(ii) Generally well answered. Some candidates successfully related the solubility of compound B to its polarity.
Question
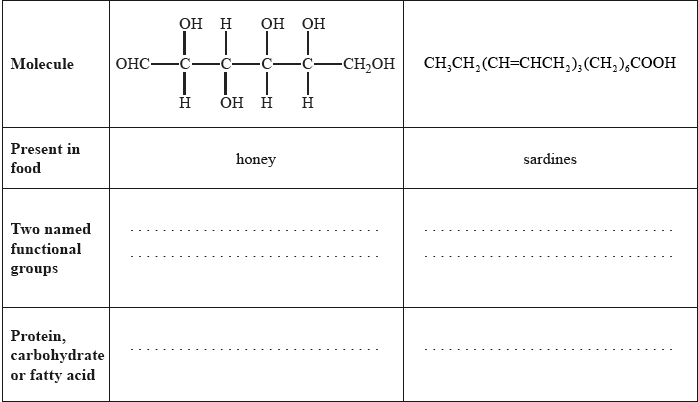
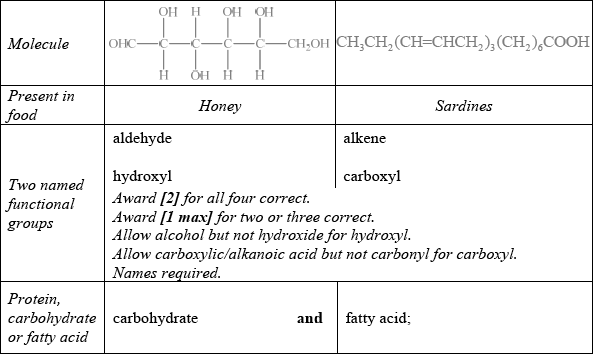
Food chemistry and nutritional science are two important scientific fields to which the general public relate.
State two named functional groups present in each of the following molecules found in two different food products (honey and sardines). Identify each molecule as a protein, a carbohydrate or a fatty acid.
Butter is an example of a saturated fat and olive oil is an example of an unsaturated fat. Describe the main structural difference between these two types of fat.
Linoleic acid, whose structure is given in Table 22 of the Data Booklet, is present in peanut oil. The oil can be converted to a semi-solid using hydrogen gas. Predict the structural formula of the compound formed from the partial hydrogenation reaction of linoleic acid, and state a suitable catalyst for this reaction.
Structural formula:
Catalyst:
Partial hydrogenation can sometimes produce trans fats. Suggest why trans fats are considered unhealthy.
Olestra, with one of its structures shown below, has been used to prepare snacks such as crisps (potato chips). Deduce the type of compound that can undergo an esterification reaction involving carboxylic acid to produce olestra.
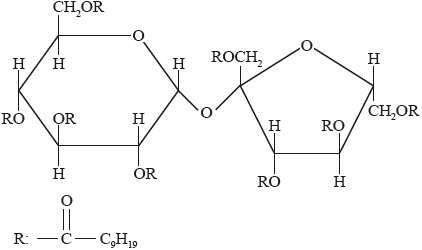
Olestra
▶️Answer/Explanation
Markscheme
Saturated fat: no carbon-carbon double bonds/no C=C/all single carbon-carbon bonds/ all C–C and Unsaturated fat: carbon-carbon double bonds/C=C/alkene groups;
Mention of carbon-carbon or alkene necessary for mark.
Structural formula:
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{CH}}\)=\({\text{CH(C}}{{\text{H}}_2}{{\text{)}}_7}{\text{COOH}}\) /
\({\text{C}}{{\text{H}}_3}{{\text{(C}}{{\text{H}}_2}{\text{)}}_4}{\text{CH}}\)=\({\text{CHC}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_2}{{\text{(C}}{{\text{H}}_2}{\text{)}}_6}{\text{COOH}}\);
Catalyst: nickel/Ni / palladium/Pd / platinum/Pt / copper/Cu / zinc/Zn;
decrease (blood) levels of HDL/high-density lipoprotein cholesterol (which protects from heart disease) / increase levels of LDL/low-density lipoprotein cholesterol (increasing risk of heart disease) / less easily digested/metabolized / leads to blocked arteries;
carbohydrate / disaccharide;
Allow sucrose / sugar.
Examiners report
Well answered with most candidates gaining two marks. The most common mistake was failing to recognize OHC– as an aldehyde.
The majority of candidates distinguished between saturated and unsaturated fats correctly.
The catalyst was usually well suggested but the structural formula was only provided by the strongest candidates. Some candidates gave the saturated product not taking note of the word “partial” in the question.
Another question better answered than in previous sessions. Many candidates related trans fats to LDL cholesterol.
This was a discriminating question. Only a few candidates recognized the compound as a carbohydrate.
Question
The principles of chromatography can be demonstrated using paper chromatography to analyse the ink of a pen, using propanone as the mobile phase.
(a) State how you could tell whether the ink was a single substance or a mixture of components.
(b) Explain how paper chromatography separates the components.
(c) The \({R_{\text{f}}}\) value of the components of the ink could be measured. Define the term \({R_{\text{f}}}\).
(d) State one factor that would alter the \({R_{\text{f}}}\) value of a particular component.
▶️Answer/Explanation
Markscheme
(a) whether chromatogram had just one spot or number of spots / OWTTE;
Allow “component/dot / OWTTE” for spot.
(b) different components have different attractions/affinities/bond strengths/solubilities for two phases / OWTTE;
components strongly absorbed/adsorbed by stationary phase move less / components weakly absorbed/adsorbed by stationary phase move more / components not very soluble in mobile phase move less / components very soluble in mobile phase move more / OWTTE;
(c) distance travelled by component divided by distance travelled by solvent (front);
Allow spot/solute for component.
Accept Rf represented as an equation.
Do not allow just retention factor.
(d) (nature of) solvent (used) / (type of) paper / temperature / pH;
Examiners report
Parts (a), (c) and (d) were well done. In (d) some candidates stated that solubility is a factor. However solubility depends on conditions and is not a factor per se. In (b) although often candidates stated that different components have different affinities for the two phases, some did not mention the two phases and others did not refer to comparative movement to score the second mark (e.g. components very soluble in the mobile phase will travel further / OWTTE). One G2 comment stated that the command term “explain” was not appropriate for this question. This was discussed at grade award and the senior examining team felt that this indeed was the most appropriate command term for the nature of the question asked.
Question
Draw the structure of a 2-amino acid.
(i) Using Table 19 of the Data Booklet, draw the structure of the two dipeptides formed by the reaction of glycine with valine.
(ii) State the other product of the reaction in (i).
Explain how a given protein can be broken down into its constituent amino acids and how these can be identified by electrophoresis.
▶️Answer/Explanation
Markscheme
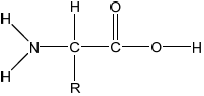 ;
;
Allow condensed structural formula (eg, R-CH(NH2)COOH) or structure of a specific 2-amino acid (eg, Ala etc.).
(i) 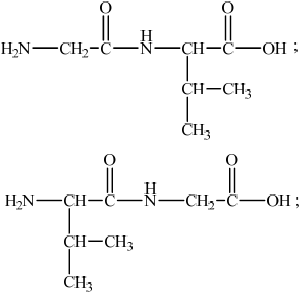
Accept condensed structural formulas.
Accept C3H7 for CH3CHCH3.
(ii) \({{\text{H}}_{\text{2}}}{\text{O}}\)/water;
add hydrochloric acid/HCl to separate individual amino acids;
Accept strong acid/concentrated H+ or restriction enzymes but not sulfuric acid/H2SO4.
Award [4 max] for any four of:
mixture/amino acids spotted/placed on gel/PAGE/polyacrylamide/paper;
gel placed in buffer/solution of known pH;
voltage/potential difference applied;
Accept “applied electric field / positive and negative electrodes connected / anode and cathode connected” but not current.
amino acids move differently depending on size and charge/isoelectric point/pH of buffer / amino acids move to oppositely charged electrodes;
Accept any suitable diagram.
develop with ninhydrin/(organic) dye / detect by UV (light)/staining/fluorescence;
Accept any suitable development method.
measure distances moved / compare with known samples / measure isoelectric points;
Examiners report
In (a) most candidates drew either the general formula of a 2-amino acid or drew the structure of a specific 2-amino acid.
In (b) (i) the structures of the two dipeptides formed by the reaction of glycine with valine were usually correctly represented and water was identified by almost all.
In (c) electrophoresis was well understood as a biochemical analytical technique. However some candidates did not read the question carefully and dropped the first mark for not stating that hydrochloric acid needed to be added to separate the individual amino acids.
Question
The building blocks of human proteins are the 2-amino acids with the general formula H2N–CHR–COOH, where R represents a side-chain specific to each amino acid. A list of these amino acids and their isoelectric points is given in table 19 of the data booklet.
State why they are called 2-amino acids.
Identify the amino acid with the empirical formula C3H7ON2
Deduce the structure of valine in a solution with a pH of 4.0.
Deduce the primary structures of the tripeptides formed by reacting together one molecule of each of the amino acids aspartic acid (Asp), glutamine (Gln) and histidine (His), using three-letter abbreviations to represent the amino acids.
Proteins carry out a number of important functions in the body. State the function of collagen.
▶️Answer/Explanation
Markscheme
NH2/amino group at C2 (while C of COOH/carboxyl/carboxylic acid is C1) / OWTTE;
Accept amine for NH2.
arginine/Arg;
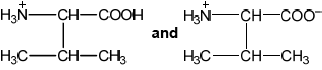 ;
;
Structural formulas of cation and zwitterion are required for the mark.
Accept structural formula of zwitterion alone (as it’s the dominant form).
Accept structural formula of cation alone (though lower in concentration than zwitterion based on equilibrium and pH calculations).
Accept full or condensed structural formula(s).
Asp–Gln–His;
Asp–His–Gln;
Gln–Asp–His;
Gln–His–Asp;
His–Asp–Gln;
His–Gln–Asp;
Award [2] for all six correct, [1] for five, four or three.
gives strength to tendons/bones/ligament/skin/cornea/cartilage/blood vessels / connective tissue;
Accept “elasticity” for “strength” but do not accept answers such as “protects bones” etc.
Accept just “structural”.
Examiners report
About a fifth of the candidates knew the reason why amino acids are called 2-amino acids. Some candidates attributed the ‘2’ to the position of the R group and others to the presence of two functional groups.
About half of the candidates were able to identify arginine as the amino acid with the empirical formula C3H7ON2.
The markscheme includes both the zwitterion and the cation, as both species exist in equilibrium at pH 4.0 (with the zwitterion having a higher concentration). Very few candidates gave the structure of the cation of valine. Some gave the structure of the zwitterion, however, the majority of candidates copied the structure of valine from the data booklet, a structure that does not exist in solution.
About a quarter of the candidates understood what was required by the question and gave the six tripeptide chains using three-letter abbreviations to represent the amino acids. Many candidates gave lots of detail for just one tripeptide.
About three quarters of the candidates stated the function of collagen correctly. Some candidates thought it provided the body with energy and others seemed to confuse it with keratin stating that it builds hair and nails.
Question
Proteins are made of long chains of amino acids.
Explain how individual amino acids can be obtained from proteins for chromatographic separation.
A mixture of amino acids was spotted onto chromatography paper and eluted with a solvent mixture. The following spots were seen after the paper had been developed with ninhydrin.
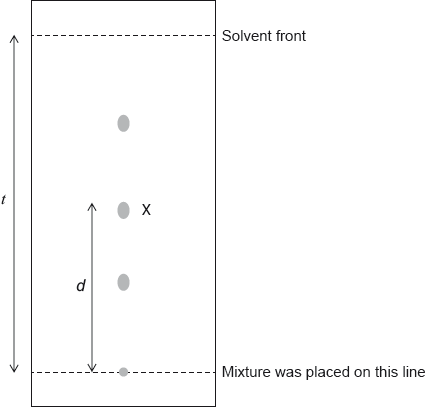
Determine the \({R_{\text{f}}}\) value of the amino acid marked as X.
One protein found in the human body is collagen. Identify its function.
▶️Answer/Explanation
Markscheme
hydrolyse/break peptide bonds;
heat/boil with (concentrated) hydrochloric acid/HCl;
Accept NaOH / enzymes.
0.50;
Accept answers in the range 0.49–0.51.
Accept d/t.
structural (protein) / connective/fibrous tissue (in tendons/ligaments/cartilage/skin) / provides elasticity/strength to skin / strengthens blood vessels;
Examiners report
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
A large number of candidates misinterpreted the question and described in detail how the process of chromatography would separate the amino acids. Candidates who interpreted the question correctly, often did not mention that the protein had to be heated with acid and did not refer to the peptide bonds being broken in this hydrolytic process.
The \({R_{\text{f}}}\) value was often not actually calculated but the correct formula for its calculation did score the mark.
Question
Amino acids, shown in section 33 of the data booklet, can be combined to form polypeptides and proteins.
Deduce the structures of the most abundant form of glycine in three buffer solutions at pH 1.0, 6.0 and 11.0.
A tripeptide, X, containing leucine (Leu), lysine (Lys) and glutamic acid (Glu) is hydrolysed and separated by gel electrophoresis in a buffer solution with a pH of 6.0.
(i) Predict the result of the electrophoresis by labeling the three spots below with the names of the amino acids.
(ii) Deduce the number of tripeptides that could be formed by using the three amino acids of tripeptide X.
▶️Answer/Explanation
Markscheme
Charges must be shown on the correct atoms in each structure for mark. Penalize repeated mistakes once.
Although question asks specifically for structures, accept condensed structural formulas, but charges must be given.
(i)
Award [2] for correct order.
Award [1 max] for Leu in centre if order is incorrect.
(ii)
6
Accept 27.
Question
Glucose, C6H12O6, is a monosaccharide that our body can use as a source of energy.
Deduce the equation for the cellular respiration of glucose.
Calculate the energy, in kJ, produced from 15.0g of glucose if its enthalpy of combustion is −2803kJmol−1.
Glucose is the basic building block of starch which can be used to make bioplastics. Outline two advantages and two disadvantages of biodegradable plastics.
Two advantages:
Two disadvantages:
Bioplastics are broken down by enzyme catalysed reactions. Sketch a graph illustrating how the rate of this reaction varies with pH.
▶️Answer/Explanation
Markscheme
C6H12O6 (aq) + 6O2 (aq) → 6CO2 (aq) + 6H2O (l)
Accept equations for anaerobic respiration, such as C6H12O6 (aq) → 2C3H6O3 (aq)
Ignore ATP if added as a product.
\(n\left( {{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}} \right)\left\langle { = \frac{{15.0}}{{180.18}}} \right\rangle = 0.0833 \ll {\rm{mol}} \gg \)
«energy=0.0833×2803=»233«kJ»
Award [2] for correct final answer.
Accept -233«kJ».
Two advantages:
renewable resource
broken down/digested by bacteria or other organisms within a relatively short time/quickly
reduce «volume of» plastic waste/landfill
reduce use of petrochemicals
OR
reduce use of fossil fuels as hydrocarbon source
degrade into non-toxic products
Any two advantages for [2 max].
M2: reference must be made to time. Do not accept “biodegradable” (since stated in question).
Ignore any mention of cost.
Two disadvantages:
require use of land «for crop production»
increased use of fertilizers/pesticides «leading to pollution»
OR
eutrophication
might break down before end of use
release of methane/CH4/greenhouse gas «during degradation»
Any two disadvantages for [2 max].
Ignore any mention of cost.
typical curve as shown in example above √
Accept any curve with a single maximum (not just bell-shaped).
Ignore features such as pH values on a pH scale or a pH value at maximum (if given).
Do not penalize if curve does not touch the x-axis.
Question
Amino acids are usually identified by their common names. Use section 33 of the data booklet.
State the IUPAC name for leucine.
A mixture of amino acids is separated by gel electrophoresis at pH 6.0. The amino acids are then stained with ninhydrin.
(i) On the diagram below draw the relative positions of the following amino acids at the end of the process: Val, Asp, Lys and Thr.
(ii) Suggest why glycine and isoleucine separate slightly at pH 6.5.
Determine the number of different tripeptides that can be made from twenty different amino acids.
The fibrous protein keratin has a secondary structure with a helical arrangement.
(i) State the type of interaction responsible for holding the protein in this arrangement.
(ii) Identify the functional groups responsible for these interactions.
▶️Answer/Explanation
Markscheme
2-amino-4-methylpentanoic acid
Accept 4-methyl-2-aminopentanoic acid.
i
Lys on cathode side AND Asp on anode side
Val at origin AND Thr on anode side but closer to origin than Asp
Val and Thr need not overlap.
Accept any (reasonable) size and demarcation of position so long as position relative to origin is correct.
Accept crosses for spots.
Award [1 max] for any two correct.
Award [1 max] if net direction of spots is reversed.
Award [1 max] if the four points are in the correct order but not in a straight line.
ii
different sizes/molar masses/chain lengths «so move with different speeds»
«203 =» 8000
i
hydrogen bonds
ii
carboxamide/amide/amido
OR
C=O AND N–H
Accept peptide.
Question
Peptidase enzyme in the digestive system hydrolyses peptide bonds.
A tripeptide Ala-Asp-Lys was hydrolysed and electrophoresis of the mixture of the amino acids was carried out at a pH of 6.0. Refer to section 33 of the data booklet.
Identify the type of metabolic process that occurs in the hydrolysis of the peptide during digestion.
Identify the name of the amino acid that does not move under the influence of the applied voltage.
Deduce, giving a reason, which amino acid will develop closest to the negative electrode.
The breakdown of a dipeptide in the presence of peptidase was investigated between 18 °C and 43 °C. The results are shown below.
Comment on the rate of reaction at temperature X in terms of the enzyme’s active site.
The solubility of a vitamin depends on its structure.
Identify the vitamin given in section 35 of the data booklet that is the most soluble in water.
Pollution from heavy metal ions has become a health concern.
Outline how the presence of heavy metal ions decreases the action of enzymes.
Outline how lead ions could be removed from an individual suffering from lead poisoning.
▶️Answer/Explanation
Markscheme
catabolism/catabolic
[1 mark]
alanine
Do not accept ala.
[1 mark]
Lys/lysine
pH «buffer» < pI «Lys»
OR
buffer more acidic than Lys «at isoelectric point»
OR
«Lys» exists as
OR
«Lys» charged positively/has +1/1+ «overall» charge «and moves to negative electrode»
Do not apply ECF from M1.
Accept converse argument.
Do not accept just “has H3N+ group” for M2 (as H3N+ is also present in zwitterion).
Do not penalize if COOH is given in the structure of lysine at pH 6 instead of COO–.
[2 marks]
highest frequency of successful collisions between active site and substrate
OR
highest frequency of collisions between active site and substrate with sufficient energy/\(E \geqslant {E_{\text{a}}}\) AND correct orientation/conformation
OR
optimal shape/conformation of the active site «that matches the substrate»
OR
best ability of the active site to bind «to the substrate»
Accept “number of collisions per unit time” for “frequency”.
Do not accept “all active sites are occupied”.
[1 mark]
ascorbic acid/vitamin C
[1 mark]
react/bind/chelate with enzyme
OR
disrupt ionic salt bridges
OR
affect shape of tertiary/quaternary structures
OR
precipitate enzymes
OR
break/disrupt disulfide bridges/bonds
Do not accept “changes shape of active site” by itself.
[1 mark]
«use of» host-guest chemistry
OR
chelation «therapy»
Accept specific medication/chelating agent such as EDTA, CaNa2 EDTA, succimer, D-penicillamine, dimercaprol.
[1 mark]
Question
The structures of the amino acids cysteine, glutamine and lysine are given in section 33 of the data booklet.
Deduce the structural formula of the dipeptide Cys-Lys.
Identify the type of bond between two cysteine residues in the tertiary structure of a protein.
Deduce the structural formula of the predominant form of cysteine at pH 1.0.
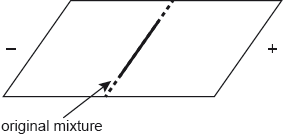
A mixture of the three amino acids, cysteine, glutamine and lysine, was placed in the centre of a square plate covered in polyacrylamide gel. The gel was saturated with a buffer solution of pH 6.0. Electrodes were connected to opposite sides of the gel and a potential difference was applied.
Sketch lines on the diagram to show the relative positions of the three amino acids after electrophoresis.
▶️Answer/Explanation
Markscheme
correct order
amide link
Accept CO–NH but not CO–HN for amide link.
Penalize incorrect bond linkages or missing hydrogens once only in 7 (a) and 7 (c).
[2 marks]
covalent
Accept “S-S/disulfide”.
[1 mark]
Penalize incorrect bond linkages or missing hydrogens once only in 7 (a) and 7 (c).
[1 mark]
Cys and Gln move to positive electrode AND Lys to negative electrode
Cys further to positive electrode than Gln
Do not penalize if lines are omitted or if different markings are given (eg, spots etc.), as long as relative positions are correctly indicated.
Accept Gln on original position indicated.
Award [1 max] for reverse order of amino acids.
[2 marks]
Question
Enzyme activity depends on many factors. Explain how pH change causes loss of activity of an enzyme.
▶️Answer/Explanation
Markscheme
conformation/shape altered
OR
active site altered
OR
tertiary structure altered
acidic/basic/ionizable/COOH/carboxyl/NH2/amino groups in the R groups/side chains «react»
exchange/lose/gain protons/H+
ionic/H-bonds altered
Accept “substrate doesn’t fit/fits poorly into active site” OR “enzyme denatures” for M1 but not “affects potential of
enzyme to form complex with substrate”.
Examiners report
Question
Insulin was the first protein to be sequenced. It was determined that the end of one chain had the primary structure Phe–Val–Asn–Gln.
Paper chromatography can be used to identify the amino acids in insulin.
Draw the structural formula of a dipeptide containing the residues of valine, Val, and asparagine, Asn, using section 33 of the data booklet.
Deduce the strongest intermolecular forces that would occur between the following amino acid residues in a protein chain.
State the name of the process used to break down the insulin protein into its constituent amino acids.
Outline how the amino acids may be identified from a paper chromatogram.
▶️Answer/Explanation
Markscheme
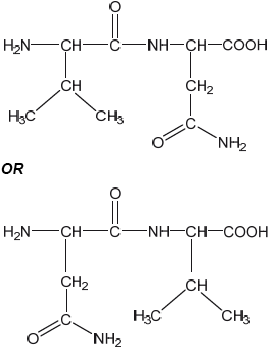
correct structures of Val AND Asn
correct amide link
[2 marks]
Phenylalanine and valine:
London/dispersion/instantaneous induced dipole-induced dipole forces
OR
permanent dipole-induced dipole «interactions»
Glutamine and asparagine:
hydrogen bonds
Do not accept dipole-dipole interactions.
[2 marks]
hydrolysis
[1 mark]
compare Rf with known amino acids
OR
compare distance moved with known amino acids
Accept “from Rf”.
[1 mark]
Question
Amino acids are the building blocks of proteins.
Draw the dipeptide represented by the formula Ala-Gly using section 33 of the data booklet.
Deduce the number of 1H NMR signals produced by the zwitterion form of alanine.
Outline why amino acids have high melting points.
▶️Answer/Explanation
Markscheme
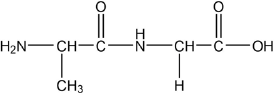
peptide bond
order of amino acids
Accept zwitterion form of dipeptide.
Accept a condensed structural formula or a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
[2 marks]
3
[1 mark]
form zwitterions
«strong» ionic bonding
OR
«strong» ionic lattice
OR
«strong» electrostatic attraction/forces
Do not accept hydrogen bonding or IMFs for M2.
[2 mark]

