Question
Outline how host–guest chemistry mimics enzymes in the removal of xenobiotics.
Answer/Explanation
Answer:
Any two of:
host molecule/supramolecule forms complex/bond with guest/xenobiotic
binding between host and guest specific
bonding «usually» non-covalent «in both cases»
Question
The amino acids in a protein can be separated using paper chromatography. The Rf values using a solvent of butanol and ethanoic acid are given.
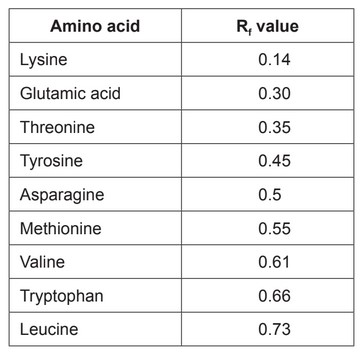
(a) The following diagram shows a chromatogram.
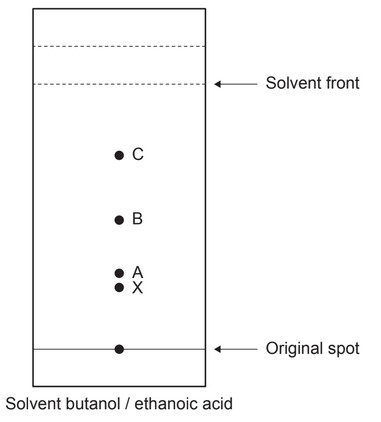
(i) Determine the identity of the amino acid creating spot C by calculating the \(R_f\) value from the chromatogram.
Identify of spot C:
(ii) Predict, referring to the structure of the amino acids, whether spot X on the chromatogram in part (a)(i) is more likely to be serine or phenylalanine. Use the table of \(R_f\) values and section 33 of the data booklet.
(b) One role of proteins in the body is to catalyse reactions. Describe how enzymes catalyse reactions in the body.
(c) State one industrial use of enzymes.
(d) Explain how a non-competitive inhibitor affects the Michaelis constant, \(K_m\), and \(V_{max}\) of a reaction. Refer to the reaction between the inhibitor and the enzyme in your answer.
Effect on \(K_m\):
Effect on \(V_{max}\):
Explanation for \(K_m\):
Explanation for \(V_{max}\)
(e) Determine the concentration, in mol \(dm^{-3}\), for a protein sample with absorbance of 0.50 at 240nm. Use section 1 of the data booklet.
Molar extinction coefficient = 0.75\(dm^3\) \(cm^{-1}\) \(mol^{-1}\)
Path length = 1.0cm
Answer/Explanation
Answer:
(a) (i) Identity of spot C:
leucine
(ii) serine AND more polar «than phenylalanine»
OR
serine AND OH group «on side chain»
hydrogen bonding/greater affinity with stationary phase
OR
less soluble/poor affinity in solvent/mobile phase
(b) bind to substrate at active site
«provide» alternative pathway with lower «activation» energy
(c) additives to detergents/washing powders/liquids
OR
breakdown oil spills/industrial waste
(d) Effect on \(K_m\): remains the same/no change AND
Effect on \(V_{max}\): decreases/reduced
Explanation for \(K_m\):
no decrease in affinity of enzyme for substrate
Explanation for \(V_{max}\):
binds at allosteric site
OR
binds away from active site
OR
changes shape of active site
OR
renders active site ineffective
(e) « \(log_{10} \frac{I_0}{I} = \varepsilon lc\) and \(log_{10}\) Io/I = A »
\(\varepsilon \) Ic = 0.50
«c = 0.50 / (0.75 \(dm^3\) \(cm^{−1}\) \(mol^{−1}\) x1 cm)»
0.67 « mol \(dm^{-3}\) »
Question
Proteins have structural or enzyme functions.
Oil spills are a major environmental problem.
a(i)Some proteins form an a-helix. State the name of another secondary protein structure.
a(ii)Compare and contrast the bonding responsible for the two secondary structures.
One similarity:
One difference:
b. Explain why an increase in temperature reduces the rate of an enzyme-catalyzed reaction.
c. State and explain how a competitive inhibitor affects the maximum rate, $V_{\max }$, of an enzyme-catalyzed reaction.
d(i)Suggest two reasons why oil decomposes faster at the surface of the ocean than at greater depth.
d(ii)il spills can be treated with an enzyme mixture to speed up decomposition.
Outline one factor to be considered when assessing the greenness of an enzyme mixture.
▶️Answer/Explanation
Markscheme
a(i) $\beta /$ beta pleated/sheet [ $\boldsymbol{C}$ ]
a(ii) One similarity:
hydrogen bonding
OR
attractions between $\mathrm{C}=\mathrm{O}$ and $\mathrm{N}-\mathrm{H}$ «on main chain» $[\boldsymbol{V}]$
One difference:
a-helix has hydrogen bonds between amino acid residues that are closer than $\beta$-pleated sheet
OR
H-bonds in a-helix parallel to helix axis AND perpendicular to sheet in $\beta$-pleated sheet
OR
a-helix has one strand $A N D$-pleated sheet has two «or more» strands
$O R$
$\alpha$-helix is more elastic «since $\mathrm{H}$-bonds can be broken easily» AND $\beta$-pleated sheet is less elastic «since $\mathrm{H}$-bonds are difficult to break» [ $\boldsymbol{\sim}$ ]
Note: Accept a diagram which shows hydrogen bonding between $\mathrm{O}$ of $\mathrm{C}=\mathrm{O}$ and $\mathrm{H}$ of $\mathrm{NH}$ groups for $\mathrm{M} 1$.
Accept “between carbonyl/amido/amide/carboxamide” but not “between amino/amine” for M1.
b. enzyme denatured/ loss of 3-D structure/conformational change
OR
«interactions responsible for» for tertiary/quaternary structures altered [ $\checkmark$ ]
shape of active site changes
OR
fewer substrate molecules fit into active sites [ $\boldsymbol{C}]$
c. $V_{\max }$ unchanged [ $\left.\sim\right]$
at high substrate concentration substrate outcompetes inhibitor/need a higher substrate concentration to reach $\mathrm{V}_{\max }$ [ $]$
Note: Accept suitable labelled diagram.
d(i)Any two of:
surface water is warmer «so faster reaction rate»/more light/energy from the sun [ $\mathcal{C}$ ]
more oxygen «for aerobic bacteria/oxidation of oil» $[\boldsymbol{\sim}]$
greater surface area $[\boldsymbol{\sim}]$
d(ii)Any one of:
non-hazardous/toxic to the environment/living organisms [ $\checkmark]$
energy requirements «during production» $[\boldsymbol{\sim}]$
quantity/type of waste produced «during production»
OR
atom economy $[\boldsymbol{\sim}]$
safety of process $[\boldsymbol{V}]$
Note: Accept “use of solvents/toxic materials “during production»”.
Do not accept “more steps involved”.
