Question
Mild heartburn is treated with antacids such as calcium carbonate.
a(i)Formulate an equation for the neutralization of stomach acid with calcium carbonate, $\mathrm{CaCO}_3$ (s).
a(iiDetermine the volume of $\mathrm{CO}_2(\mathrm{~g})$, in $\mathrm{dm}^3$, produced at $\mathrm{STP}$, when $1.00 \mathrm{~g}$ of $\mathrm{CaCO}_3$ (s) reacts completely with stomach acid.
$$
M_r \mathrm{CaCO}_3=100.09
$$
b. Acid secretion can be regulated by other types of drugs such as omeprazole and ranitidine. Outline how each of these drugs acts to reduce excess stomach acid.
Omeprazole:
Ranitidine:
▶️Answer/Explanation
Markscheme
$$
\mathrm{a}(\mathrm{i}) \mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+\mathrm{CaCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})[\boldsymbol{\sim}]
$$
Note: Accept balanced ionic equations involving ” $\mathrm{H}^{+}$” or ” $\mathrm{H}_3 \mathrm{O}^{+ \text {“. }}$
Do not accept ” $\mathrm{H}_2 \mathrm{CO}_3$ “.
$$
\begin{aligned}
& \text { a(ii) } \mathrm{CaCO}_3=« \frac{1.00 \mathrm{~g}}{100.09 \mathrm{~g} \mathrm{~mol}^{-1}}=0.00999 \text { «mol» }[\boldsymbol{\sim}] \\
& \text { volume } \mathrm{CO}_2=« 0.00999 \mathrm{~mol} \times 22.7 \mathrm{dm}^3 \mathrm{~mol}^{-1}=» 0.227 « \mathrm{dm}^3 »[\boldsymbol{V}] \\
&
\end{aligned}
$$
Note: Accept 0.224 «dm 3 » if $22.4 \mathrm{dm}^3 \mathrm{~mol}^{-1}$ is used as molar volume.
Award [2] for correct answer.
b. Omeprazole:
inhibits enzyme/«gastric» proton pump «which secretes $\mathrm{H}^{+}$ions into gastric juice»
OR
inhibits the $\mathrm{H}^{+} / \mathrm{K}^{+}$-ATPase system [ $\left.\boldsymbol{V}\right]$
Ranitidine:
inhibits/blocks $\mathrm{H}$ 2/histamine receptors «in cells of stomach lining»
OR
prevents histamine binding to $\mathrm{H} 2$ /histamine receptors «and triggering acid secretion» [ $\boldsymbol{C}$
Note: Accept “H2-receptor antagonist” for M2.
Question
Excess acid in the stomach can cause breakdown of the stomach lining.
a(i)Outline how ranitidine (Zantac) inhibits stomach acid production.
a(ii)utline two advantages of taking ranitidine instead of an antacid which neutralizes excess acid.
b. Some antacids contain carbonates.
Determine the $\mathrm{pH}$ of a buffer solution which contains $0.160 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{CO}_3{ }^{2-}$ and $0.200 \mathrm{~mol} \mathrm{dm}^{-3} \mathrm{HCO}_3{ }^{-}$, using section 1 of the data booklet. $\mathrm{pK}_{\mathrm{a}}\left(\mathrm{HCO}_3{ }^{-}\right)=10.32$
▶️Answer/Explanation
Markscheme
a(i)blocks/binds $\mathrm{H} 2 /$ histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to $\mathrm{H} 2 /$ histamine receptors «and triggering acid secretion» [ $]$
a(ii)Any two of:
ranitidine can be effective in treating ulcers «but antacid is not» $[\boldsymbol{C}]$
ranitidine can prevent long-term damage «from overproduction of acid and antacid does not» [ $\boldsymbol{V}]$
ranitidine has a long-term effect «and antacid has short-term effect only» $[\boldsymbol{\sim}]$
ranitidine does not affect ionic balance in body «and antacid does» $[\boldsymbol{\sim}]$
ranitidine does not produce bloating/flatulence $[\boldsymbol{\sim}]$
Note: Accept “ranitidine stops the over production of acid in the stomach while antacids neutralize the excess acid giving temporary relief”.
b. $« \mathrm{pH}=\mathrm{p} K_a+\log \frac{\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}=10.32+\log \frac{0.160}{0.200}=10.32-0.097 »$
$$
« \mathrm{pH}=» 10.22[\boldsymbol{C}]
$$
Question
Body fluids have different $\mathrm{pH}$ values.
a. Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
b. An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
c. Outline how ranitidine reduces stomach acidity.
d. Calculate the $\mathrm{pH}$ of a buffer solution which contains $0.20 \mathrm{~mol} \mathrm{dm}^{-3}$ ethanoic acid and $0.50 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium ethanoate. Use section 1 of the data booklet.
$\mathrm{p} K_{\mathrm{a}}($ ethanoic acid $)=4.76$
▶️Answer/Explanation
Markscheme
a. hydrochloric acid/ $\mathrm{HCl}$ «(aq)» $A N D$ strong «acid»
b. $\mathrm{MgCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{MgCl}_2(\mathrm{aq})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}$
NOTE: Accept ionic equation.
c. blocks/binds to H2-histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to $\mathrm{H} 2$-histamine receptors «and triggering acid secretion»
OR
prevents parietal cells from releasing/producing acid
NOTE: Do not accept “antihistamine” by itself.
Accept “H2-receptor antagonist/H2RA” OR “blocks/inhibits action of histamine”.
Accept “blocks receptors in parietal cells “from releasing/producing acid»”.
Do not accept “proton pump/ATPase inhibitor”.
d. $« p K_{\mathrm{a}}=4.76 »$
$$
\begin{aligned}
& \text { «pH }=\mathrm{p} K_{\mathrm{a}}+\log \left(\frac{\left[\mathrm{CH}_3 \mathrm{COO}^{-}\right]}{\left[\mathrm{CH}_3 \mathrm{COOH}\right]}\right) » \\
& « \mathrm{pH}=4.76+0.40=» 5.16
\end{aligned}
$$
Question
Buffer systems control pH in the body.
a. Determine the $\mathrm{pH}$ of a buffer solution that is $0.0100 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium hydrogen carbonate and $0.0200 \mathrm{~mol} \mathrm{dm}^{-3}$ sodium carbonate, using section 1 of the data booklet.
$K_{\mathrm{a}}($ hydrogen carbonate ion $)=4.8 \times 10^{-11}$
b. State the equation for the reaction of calcium carbonate, the active ingredient in some antacids, with stomach acid.
c. Suggest a technique for measuring the percentage mass of calcium carbonate in this type of antacid tablet.
▶️Answer/Explanation
Markscheme
a. ALTERNATIVE 1:
Using: $p H=p K_a+\log \left(\frac{\left[\mathrm{A}^{-}\right]}{[\mathrm{HA}]}\right)$
$$
\begin{aligned}
& p K_{\mathrm{a}}=10.32 \\
& \mathrm{pH}=\ll 10.32+\log \left(\frac{0.0200}{0.0100}\right)=» 10.62
\end{aligned}
$$
ALTERNATIVE 2:
$$
\begin{aligned}
& {\left[\mathrm{H}^{+}\right] \lll=K_{\mathrm{a}} \times\left(\frac{0.0100}{0.0200}\right) »=2.4 \times 10^{-11}} \\
& \mathrm{pH}=10.62
\end{aligned}
$$
Award [2] for correct final answer.
Accept answers for M2 between 10.6 and 10.7.
Award [1 max] for $\mathrm{pH}=10.02$.
b. $\mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{CaCl}_2(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})$
OR
$$
\mathrm{CaCO}_3(\mathrm{~s})+2 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow \mathrm{Ca}^{2+}(\mathrm{aq})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})
$$
c. «back» titration
OR
thermal decomposition
OR
atomic absorption/AA
Accept “gravimetric analysis”.
Do not accept description of a technique without proper term given for the technique.
Question
Excess acid in the stomach is often treated with calcium carbonate.
a. Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
b. Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing $0.750 \mathrm{~g}$ calcium carbonate.
c. Explain how omeprazole (Prilosec) regulates $\mathrm{pH}$ in the stomach.
▶️Answer/Explanation
Markscheme
a. $2 \mathrm{HCl}(\mathrm{aq})+\mathrm{CaCO}_3(\mathrm{~s}) \rightarrow \mathrm{H}_2 \mathrm{O}(\mathrm{l})+\mathrm{CO}_2(\mathrm{~g})+\mathrm{CaCl}_2(\mathrm{aq})$
Accept ionic equation:
$2 \mathrm{H}^{+}(\mathrm{aq})+\mathrm{CO}_3{ }^{2-}(\mathrm{aq}) \rightarrow \mathrm{CO}_2(\mathrm{~g})+\mathrm{H}_2 \mathrm{O}(\mathrm{l})$
[1 mark]
b. $« \frac{0.750 \times 2}{100.09}=0.0150$ «mol HCl»
[1 mark]
c. inhibits the secretion of stomach acid/ $/ \mathrm{H}^{+}$
«active metabolites» bind «irreversibly» to «receptors of the» proton pump
Do not accept “hydrogen/ $\mathrm{H} / \mathrm{H}_2$ ” for ” $\mathrm{H}^{+}$”.
Accept “PPI/proton pump inhibitor” for M2.
Accept ” $H^{+} / K^{+}$ATPase” for “proton pump”.
[2 marks]
Question
The structures of oseltamivir (Tamiflu) and zanamivir (Relenza) are given in section 37 of the data booklet.
a.i. Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
a.iiDeduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
b. Suggest one ethical consideration faced by medical researchers when developing medications.
▶️Answer/Explanation
Markscheme
a.i. One similarity:
both contain amido «group»
One difference:
oseltamivir contains ester «group» AND zanamivir does not
OR
oseltamivir contains amino «group» $A N D$ zanamivir does not «but contains a guanidino group» OR
zanamivir contains carboxyl «group» AND oseltamivir does not
OR
zanamivir contains «several» hydroxyl «groups» $A N D$ oseltamivir does not
OR
oseltamivir contains ester «group» AND zanamivir contains carboxyl «group»
OR
oseltamivir contains ester «group» $A N D$ zanamivir contains «several» hydroxyl «groups»
Accept “both contain ether “group»” OR “both contain alkene/alkenyl “group»” OR “both contain carbonyl “group»” OR “both contain amino/amine “group»”. Latter cannot be given in combination with second difference alternative with respect to amino group.
Accept “amide/carboxamide/carbamoyl” for “amido”.
Accept “amine” for “amino”.
Accept “carboxylic acid” for “carboxyl”.
Accept “hydroxy/alcohol” for “hydroxyl”, but not “hydroxide”.
[2 marks]
a.ii.1050-1410
OR
1620-1680
OR
1700-1750
OR
2500-3000
OR
3200-3600
OR
2850-3090
$O R$
$3300-3500 \ll \mathrm{cm}^{-1} »$
[1 mark]
b. «negative» side-effects of medication on patient/volunteers
OR
effects on environment «from all materials used and produced»
OR
potential for abuse
OR
drugs may be developed that are contrary to some religious doctrines
OR
animal testing
OR
risk to benefit ratio
OR
appropriate consent of patient volunteers
[1 mark]
Question
Excess stomach acid leads to medical conditions that affect many people worldwide. These conditions can be treated with several types of medical drugs.
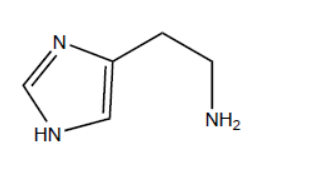
a. Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
b. Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
c. A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, $\mathrm{H}^{+}$, per mole of antacid.
▶️Answer/Explanation
Markscheme
a. «ranitidine» blocks/inhibits histamine binding to «H2» receptor
OR
ranitidine binds to same «H2» receptors «as histamine»
OR
competes with histamine for binding
b. proton pump
OR
$\mathrm{H}^{+} / \mathrm{K}^{+}$ATPase enzyme
Accept “«secretary surface of» parietal cells”.
Do not accept “stomach/stomach wall”.
c. $\mathrm{Al}(\mathrm{OH})_3(\mathrm{~s})+3 \mathrm{H}^{+}(\mathrm{aq}) \rightarrow \mathrm{Al}^{3+}(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})$
OR
$$
\mathrm{Al}(\mathrm{OH})_3(\mathrm{~s})+3 \mathrm{HCl}(\mathrm{aq}) \rightarrow \mathrm{AlCl}_3(\mathrm{aq})+3 \mathrm{H}_2 \mathrm{O}(\mathrm{l})
$$
Question
The walls of the human stomach contain cells that produce gastric juices. Sodium hydrogencarbonate is an antacid often used to neutralize excess acid.
State an equation for the reaction of stomach acid with this antacid.
Calcium carbonate can also neutralize stomach acid. The same amounts (in moles) of sodium hydrogencarbonate and calcium carbonate are available. Deduce which antacid will neutralize the greater amount of acid present in the stomach and explain your reasoning.
▶️Answer/Explanation
Markscheme
\({\text{NaHC}}{{\text{O}}_{\text{3}}} + {\text{HCl}} \to {\text{NaCl}} + {{\text{H}}_{\text{2}}}{\text{O}} + {\text{C}}{{\text{O}}_{\text{2}}}/{\text{HCO}}_3^– + {{\text{H}}^ + } \to {{\text{H}}_{\text{2}}}{\text{O}} + {\text{C}}{{\text{O}}_{\text{2}}}\);
States not required for mark.
\({\text{CaC}}{{\text{O}}_{\text{3}}}\);
1 mol \({\text{NaHC}}{{\text{O}}_{\text{3}}}\) neutralizes 1 mol HCl and 1 mol \({\text{CaC}}{{\text{O}}_{\text{3}}}\) neutralizes 2 mol HCl / \({\text{CaC}}{{\text{O}}_{\text{3}}} + {\text{2HCl}} \to {\text{CaC}}{{\text{l}}_{\text{2}}} + {{\text{H}}_{\text{2}}}{\text{O}} + {\text{C}}{{\text{O}}_{\text{2}}}\);
Examiners report
Most candidates gave correct equations.
Most candidates identified \({\text{CaC}}{{\text{O}}_{\text{3}}}\) as the antiacid that neutralizes more acid.
Question
Sodium hydrogencarbonate, NaHCO3, and magnesium hydroxide, Mg(OH)2, can both be used as antacids.
(i) Give the equations for the reactions of sodium hydrogencarbonate and magnesium hydroxide with hydrochloric acid.
(ii) Compare the effectiveness of 1.00 g of sodium hydrogencarbonate to 0.50 g of magnesium hydroxide in combating acidity in the stomach.
▶️Answer/Explanation
Markscheme
(i) \({\text{NaHC}}{{\text{O}}_{\text{3}}} + {\text{HCl}} \to {\text{NaCl}} + {{\text{H}}_{\text{2}}}{\text{O}} + {\text{C}}{{\text{O}}_{\text{2}}}\);
Accept H2 CO3 instead of H2O and CO2.
\({\text{Mg(OH}}{{\text{)}}_2} + {\text{2HCl}} \to {\text{MgC}}{{\text{l}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}}\);
(ii) \({\text{n(NaHC}}{{\text{O}}_{\text{3}}}{\text{)}} = 1.19 \times {10^{ – 2}}{\text{ mol}}\);
\({\text{n}}\left( {{\text{Mg(OH}}{{\text{)}}_{\text{2}}}} \right) = 8.37 \times {10^{ – 3}}{\text{ mol}}\);
\({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\) reacts with twice the number of moles of acid / is more effective than \({\text{NaHC}}{{\text{O}}_{\text{3}}}\) / OWTTE;
Examiners report
Most candidates successfully wrote balanced equations for antacid reactions in part (a), although a few didn’t know the products, some didn’t balance the equations and many candidates incorrectly wrote the formula for magnesium chloride as MgCl. This led to difficulties in comparing the effectiveness of two antacids, with several candidates not even attempting to answer the question. Some candidates interpreted the coefficients in the equations as representing the mass ratio rather than a mole ratio.
Question
Dyspepsia, commonly known as indigestion, is due to excess acid in the stomach and can be treated using antacids.
State the name of the acid found in the gastric juices of the stomach.
Two examples of antacids are aluminium hydroxide and calcium carbonate. State the equations to show the action of each antacid.
▶️Answer/Explanation
Markscheme
hydrochloric acid;
\({\text{Al(OH}}{{\text{)}}_{\text{3}}} + {\text{3HCl}} \to {\text{AlC}}{{\text{l}}_{\text{3}}} + {\text{3}}{{\text{H}}_{\text{2}}}{\text{O}}\);
\({\text{CaC}}{{\text{O}}_3} + {\text{2HCl}} \to {\text{CaC}}{{\text{l}}_2} + {\text{C}}{{\text{O}}_2} + {{\text{H}}_2}{\text{O}}\);
Ignore state symbols.
Award [1 max] for correct reactants and products in both equations if equations are not balanced.
Examiners report
The vast majority scored the mark in (a), but a small number of candidates gave the formula for hydrochloric acid rather than writing the name.
In (b) many correct answers were given but it was surprising to see that some candidates did not know the correct chemical formulas and how to balance equations. In some cases candidates wrote an equation for the reaction between aluminium hydroxide and calcium carbonate.
Question
Two substances commonly used in antacid tablets are magnesium hydroxide and aluminium hydroxide.
State an equation to represent a neutralization reaction with one of the above antacids.
State and explain whether 0.1 mol of magnesium hydroxide is more effective or less effective than 0.1 mol of aluminium hydroxide.
Suggest why compounds such as sodium hydroxide or potassium hydroxide cannot be used as an antacid.
▶️Answer/Explanation
Markscheme
\({\text{Al(OH}}{{\text{)}}_3} + {\text{3HCl}} \to {\text{AlC}}{{\text{l}}_3} + {\text{3}}{{\text{H}}_2}{\text{O}}/{\text{Mg(OH}}{{\text{)}}_2} + {\text{2HCl}} \to {\text{MgC}}{{\text{l}}_2} + {\text{2}}{{\text{H}}_2}{\text{O}}\);
Accept ionic equations.
less effective and (magnesium hydroxide) 2/0.2 mol \({\text{O}}{{\text{H}}^ – }\) ions available as compared to (aluminium hydroxide) 3/0.3 mol OH– ions for neutralization / neutralizes \({\text{2}}{{\text{H}}^ + }\)/0.2 mol acid as compared to \({\text{3}}{{\text{H}}^ + }\)/0.3 mol acid; [1]
Do not accept aluminium hydroxide can neutralize more acid.
strong base / corrosive / harmful to the body;
Examiners report
Whilst quite a few candidates could write one of the required balanced equations a surprising large number could not succeed in this simple task. Answers to the second part of this question often lacked the stoichiometric rigour required and the reasons for not using strong alkalis provoked an amazing range of responses, mostly incorrect and many exposing a worrying lack of basic chemical knowledge. Only a minority of the students could correctly identify the function of alginates and dimethicone in antacid preparations.
Question
A well-known brand of antacids contains 0.160 g of aluminium hydroxide and 0.105 g of magnesium carbonate in each tablet.
State the separate equations for the reactions of aluminium hydroxide and magnesium carbonate with hydrochloric acid.
Determine which of the two components of the tablet will neutralize the most acid.
On the leaflet which comes with the tablets it states that one of the side effects of the tablets is belching. Explain why this might occur.
▶️Answer/Explanation
Markscheme
\({\text{Al(OH}}{{\text{)}}_3} + {\text{3HCl}} \to {\text{AlC}}{{\text{l}}_3} + {\text{3}}{{\text{H}}_2}{\text{O}}\);
\({\text{MgC}}{{\text{O}}_3} + {\text{2HCl}} \to {\text{MgC}}{{\text{l}}_2} + {{\text{H}}_2}{\text{O}} + {\text{C}}{{\text{O}}_2}\);
\(n\,{\text{Al(OH}}{{\text{)}}_3} = \frac{{0.160}}{{77.95}} = 2.05 \times {10^{ – 3}}{\text{ (mol)}}\)
and \(n\,{\text{MgC}}{{\text{O}}_{\text{3}}} = \frac{{0.105}}{{84.32}} = 1.25 \times {10^{ – 3}}{\text{ (mol)}}\);
Do not penalize use of integer values for \({M_r}\).
\({\text{Al(OH}}{{\text{)}}_3}\) neutralizes \({\text{6.15}} \times {\text{1}}{{\text{0}}^{ – 3}}{\text{ mol}}\) of acid and
\({\text{MgC}}{{\text{O}}_{\text{3}}}\) neutralizes \(2.50 \times {10^{ – 3}}{\text{ mol}}\) of acid;
due to carbon dioxide (from reaction of \({\text{MgC}}{{\text{O}}_{\text{3}}}{\text{/NaHC}}{{\text{O}}_{\text{3}}}\) with acid);
Examiners report
Most candidates were very familiar with at least one of the two equations. Some did not read the question carefully and stated the equation for magnesium hydroxide instead of magnesium carbonate.
Unfortunately many lost points in (b) as they did not carry through the calculations corresponding to the provided data and some did not realize that a calculation was required.
Part (d) was mostly correctly answered.
Question
Aluminium hydroxide and calcium carbonate are both used as antacids.
State an equation for the reactions that occur in the stomach for both substances with hydrochloric acid.
Aluminium hydroxide:
Calcium carbonate:
A typical antacid tablet has a mass of about 1 g. Determine which of the two antacids will neutralize the greater amount of hydrochloric acid if tablets of each are added to separate samples of acid. A detailed calculation is not required.
Potassium hydroxide also neutralizes hydrochloric acid. Suggest why it is not used as an antacid.
▶️Answer/Explanation
Markscheme
\({\text{Al(OH}}{{\text{)}}_3}{\text{(s)}} + {\text{3HCl(aq)}} \to {\text{AlC}}{{\text{l}}_3}{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\);
\({\text{CaC}}{{\text{O}}_3}{\text{(s)}} + {\text{2HCl(aq)}} \to {\text{CaC}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Ignore state symbols.
\({\text{Al(OH}}{{\text{)}}_{\text{3}}}\) has smaller molar mass (so more moles per tablet);
one mole of \({\text{Al(OH}}{{\text{)}}_{\text{3}}}\) neutralizes more moles of acid;
OR
(for Al(OH)3) \({n_{^{{\text{HCL}}}}} = \frac{3}{{78}}{\text{ (mol)}}\);
(for CACO3) \({n_{^{{\text{HCL}}}}} = \frac{2}{{100}}{\text{ (mol)}}\);
strong (soluble) base/alkali;
damage to/corrosive to body/tissue;
Examiners report
There were various interpretations of the formula of aluminium hydroxide in (a) and many were able to gain one mark, the stoichiometric mark, in (b). Many did not realize that molar mass is also of consequence. Part (c) was a source of concern with many candidates showing an absolute lack of knowledge of potassium hydroxide and its chemical nature. Indeed, many seemed to think that we were asking about potassium itself! One common answer was that KOH only neutralizes one mole of HCl. The answers to (d) were either very good or somewhat vague and lacked specific reference to how placebos are used in drug development.
Question
Maalox® manufactures several different types of antacid. Maalox® Extra Strength is a suspension. One teaspoon (\({\text{5.00 c}}{{\text{m}}^{\text{3}}}\)) contains 400 mg of magnesium hydroxide, \({\text{Mg(OH}}{{\text{)}}_{\text{2}}}\), 306 mg of aluminium hydroxide, \({\text{Al(OH}}{{\text{)}}_{\text{3}}}\), and 40.0 mg of simethicone. Maalox® Extra Strength with Anti-gas comes in tablet form. Each tablet contains 1000 mg of calcium carbonate, \({\text{CaC}}{{\text{O}}_{\text{3}}}\), and 60.0 mg of simethicone.
Stomach acid approximates to \(1.00 \times {10^{ – 2}}{\text{ mol}}\,{\text{d}}{{\text{m}}^{ – 3}}\) hydrochloric acid. Assuming that simethicone does not react with acid, determine the volume, in \({\text{d}}{{\text{m}}^{\text{3}}}\), of stomach acid neutralized by:
State the equations for the reactions of magnesium hydroxide, aluminium hydroxide and calcium carbonate with hydrochloric acid.
Magnesium hydroxide:
Aluminium hydroxide:
Calcium carbonate:
(i) one teaspoon (\({\text{5.00 c}}{{\text{m}}^{\text{3}}}\)) of Maalox® Extra Strength.
(ii) one tablet of Maalox® Extra Strength with Anti-gas.
▶️Answer/Explanation
Markscheme
\({\text{Mg(OH}}{{\text{)}}_{\text{2}}} + {\text{2HCl}} \to {\text{MgC}}{{\text{l}}_{\text{2}}} + {\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}\);
\({\text{Al(OH}}{{\text{)}}_3} + {\text{3HCl}} \to {\text{AlC}}{{\text{l}}_3} + {\text{3}}{{\text{H}}_2}{\text{O}}\);
\({\text{CaC}}{{\text{O}}_3} + {\text{2HCl}} \to {\text{CaC}}{{\text{l}}_2} + {{\text{H}}_2}{\text{O}} + {\text{C}}{{\text{O}}_2}\);
Accept H2CO3 for H2O and CO2.
(i) amount of \({\text{Mg(OH}}{{\text{)}}_2} = \left( {\frac{{0.400}}{{(24.31 + 32.00 + 2.02)}} = \frac{{0.400}}{{58.33}} = } \right){\text{ }}6.86 \times {10^{ – 3}}{\text{ (mol)}}\)
and amount of \({\text{Al(OH}}{{\text{)}}_3} = \left( {\frac{{0.306}}{{(26.92 + 48.00 + 3.03)}} = \frac{{0.306}}{{77.95}} = } \right){\text{ }}3.93 \times {10^{ – 3}}{\text{ (mol)}}\);
amount of HCl reacting \( = (2 \times 6.86 \times {10^{ – 3}}) + (3 \times 3.93 \times {10^{ – 3}}) = 2.55 \times {10^{ – 2}}{\text{ (mol)}}\) so volume of \(1.00 \times {10^{ – 2}}{\text{ HCl}} = 2.55{\text{ }}({\text{d}}{{\text{m}}^3})\);
No ECF from (a) if formulas of Mg(OH)2 or Al(OH)3 are incorrect.
Allow integer values for atomic masses.
Award [2] for correct final answer.
(ii) amount of \({\text{CaC}}{{\text{O}}_3} = \left( {\frac{{1.000}}{{(40.08 + 12.01 + 48.00)}} = \frac{{1.000}}{{100.09}} = } \right){\text{ }}9.99 \times {10^{ – 3}}{\text{ (mol)}}\);
amount of HCl reacting \( = (2 \times 9.99 \times {10^{ – 3}}) = 2.00 \times {10^{ – 2}}{\text{ (mol)}}\) so volume of \(1.00 \times {10^{ – 2}}{\text{ HCl}} = 2.00{\text{ }}({\text{d}}{{\text{m}}^3})\);
Allow integer values for atomic masses.
Award [2] for correct final answer.
Penalize incorrect answer based on same units mistake once only in 12 (b) (i) and (ii).
Examiners report
Majority of the candidates were able to provide correct balanced equations for part (a) and score the three marks. Many were able to score one mark for completing the calculations in part (b)(i). The most common errors were using the incorrect value for the volume in step 2 or deducing the incorrect amount of HCl reacting. Most candidates performed well on part (b)(ii). For part (c) candidates lost the mark for stating it prevents flatulence.
Question
Adults can produce approximately \({\text{2 d}}{{\text{m}}^{\text{3}}}\) of gastric juice daily in the stomach.
The pH of gastric juice is 1.5. Identify the compound responsible for its acidity and state whether it is a strong or weak acid.
Compound:
Strong or weak acid:
Antacid tablets are often taken for an upset stomach. Identify the reaction involved in this treatment and state the general ionic equation for this reaction type.
Type of reaction:
Ionic equation:
One active ingredient in a commercial brand of antacid tablets is a complex of aluminium hydroxide and sodium carbonate, dihydroxyaluminium sodium carbonate, \({\text{Al(OH}}{{\text{)}}_{\text{2}}}{\text{NaC}}{{\text{O}}_{\text{3}}}{\text{(s)}}\).
Deduce the balanced equation, including state symbols, for the reaction of \({\text{Al(OH}}{{\text{)}}_{\text{2}}}{\text{NaC}}{{\text{O}}_{\text{3}}}{\text{(s)}}\) with the acid present in gastric juice.
▶️Answer/Explanation
Markscheme
Compound:
hydrochloric acid/HCl;
Strong or weak acid:
strong (acid);
Type of reaction:
neutralization;
Accept acid-base.
Ionic equation:
\({{\text{H}}^ + }{\text{(aq)}} + {\text{O}}{{\text{H}}^ – }{\text{(aq)}} \to {{\text{H}}_2}{\text{O(l)}}/{\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{CO}}_3^{2 – }{\text{(aq)}} \to {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\) /
\({{\text{H}}^ + }{\text{(aq)}} + {\text{HCO}}_3^ – {\text{(aq)}} \to {{\text{H}}_2}{\text{O(l)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}}\);
Accept equations such as Mg(OH)2(s) + 2H+(aq) \( \to \) Mg2+(aq) + 2H2O(l).
Ignore state symbols.
H3O+ or H+ may be used in the equation.
Do not allow the inclusion of spectator ions.
\({\text{Al(OH}}{{\text{)}}_2}{\text{NaC}}{{\text{O}}_3}{\text{(s)}} + {\text{4HCl(aq)}} \to {\text{AlC}}{{\text{l}}_3}{\text{(aq)}} + {\text{NaCl(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\) /
\({\text{Al(OH}}{{\text{)}}_2}{\text{NaC}}{{\text{O}}_3}{\text{(s)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{A}}{{\text{l}}^{3 + }}{\text{(aq)}} + {\text{N}}{{\text{a}}^ + }{\text{(aq)}} + {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}}\);
correct reactants and products;
correct state symbols and balanced;
M2 can only be awarded if M1 is correct.
Examiners report
Very well answered by most candidates recognizing the acid in the stomach as hydrochloric acid and that it is a strong acid.
Surprisingly only about half of the candidates recognized the reaction of antacids in the stomach as a neutralization reaction and only few candidates gave a correct ionic equation for the reaction.
This was a discriminating question. Only the strong candidates were able to identify the correct products scoring one mark, and very few were able to add the correct state symbols and balance the equation. Common mistakes included writing solid state symbols for the salt products and writing an incorrect formula of aluminium chloride such as \({\text{AlC}}{{\text{l}}_{\text{2}}}\). A number of candidates had aluminium hydroxide as a product.
Question
Magnesium hydroxide is the active ingredient in a common antacid.
Formulate the equation for the neutralization of stomach acid with magnesium hydroxide.
Determine the mass of HCl, in g, that can be neutralized by the standard adult dose of 1.00g magnesium hydroxide.
Compare and contrast the use of omeprazole (Prilosec) and magnesium hydroxide.
▶️Answer/Explanation
Markscheme
Mg (OH)2(s) + 2HCl (aq) → 2H2O (l) + MgCl2 (aq)
OR
Mg (OH)2 (s) + 2H+ (aq) → Mg2+ (aq) + 2H2O (l)
\(\frac{{1.00}}{{58.33}}\)=0.0171«molMg(OH)2»
«0.0171×2×36.46=»1.25«g»
Award [2] for 1.25 or 1.26 «g».
Award [1 max] for any similarity:
both compounds relieve symptoms of acid reflux/heartburn/indigestion
OR
both increase the stomach pH
both cause diarrhoea
Award [2 max] for any two differences:
omeprazole stops the production of acid/is a proton-pump inhibitor AND magnesium hydroxide neutralizes the «excess» acid that is present
omeprazole takes longer «than magnesium hydroxide» to provide relief
omeprazole is used to treat ulcers while magnesium hydroxide is not
omeprazole can prevent long term damage from overproduction of acid AND magnesium hydroxide does not
OR
omeprazole has a long term effect AND magnesium hydroxide has a short-term effect «only»
magnesium hydroxide affects ionic balance in the body AND omeprazole does not
Award [1 max] if two or three correct points are given about one of the compounds without addressing the other compound.
Question
Excess stomach acid leads to medical conditions that affect many people worldwide. These conditions can be treated with several types of medical drugs.
Ranitidine (Zantac) is a drug that inhibits stomach acid production. Outline why the development of this drug was based on a detailed knowledge of the structure of histamine, shown below.
Two other drugs, omeprazole (Prilosec) and esomeprazole (Nexium), directly prevent the release of acid into the stomach. Identify the site of action in the body.
A different approach to treating excess stomach acid is to neutralize it with antacids. Formulate an equation that shows the action of an antacid that can neutralize three moles of hydrogen ions, H+, per mole of antacid.
▶️Answer/Explanation
Markscheme
«ranitidine» blocks/inhibits histamine binding to «H2» receptor
OR
ranitidine binds to same «H2» receptors «as histamine»
OR
competes with histamine for binding
proton pump
OR
H+ /K+ ATPase enzyme
Accept “«secretary surface of» parietal cells”.
Do not accept “stomach/stomach wall”.
Al(OH)3(s) + 3H+ (aq) → Al3+ (aq) + 3H2O (l)
OR
Al(OH)3(s) + 3HCl (aq) → AlCl3 (aq) + 3H2O (l)
Question
The buffer formed by carbon dioxide, CO2(aq) and hydrogen carbonate ion, HCO3−(aq), plays an important role in maintaining the pH of blood.
Calculate the pH of the buffer from the following data and section 1 of the data booklet.
pKa(CO2) = 6.34
[HCO3−(aq)] = 1.40 × 10−2 mol\(\,\)dm−3
[CO2(aq)] = 1.25 × 10−3 mol\(\,\)dm−3
Explain the effect of a large amount of aspirin on the pH of blood.
▶️Answer/Explanation
Mark scheme
«pH = pKa + log10 \(\left( {\frac{{\left[ {{\text{HC}}{{\text{O}}_3}^ – } \right]}}{{\left[ {{\text{C}}{{\text{O}}_2}} \right]}}} \right) = 6.34 + {\log _{10\;}}(11.2) = 6.34 + 1.05\)» = 7.39
[1 mark]
H+ from aspirin reacts with HCO3− to form CO2 and H2O
OR
H+(aq) + HCO3−(aq) \( \rightleftharpoons \) CO2(aq) + H2O(l)
OR
reverse reaction favoured «to use up some of the H+ added»
pH decreases
No mark for “stating aspirin is a weak acid that dissociates partially to produce H+” without reference to reaction with HCO3− or to the equation.
Reversible arrows not required for the mark.
Do not accept “small pH change when small amount of H+ is added”.
[2 marks]
Question
A number of drugs have been developed to treat excess acidity in the stomach.
Two drugs are ranitidine (Zantac) and omeprazole (Prilosec). Outline how they function to reduce stomach acidity.
0.500 g of solid anhydrous sodium carbonate, Na2CO3(s), is dissolved in 75.0 cm3 of 0.100 mol\(\,\)dm−3 sodium hydrogen carbonate solution, NaHCO3(aq). Assume the volume does not change when the salt dissolves.
HCO3−(aq) \( \rightleftharpoons \) CO32−(aq) + H+(aq) pKa = 10.35.
Calculate the pH of the buffer solution.
▶️Answer/Explanation
Markscheme
Ranitidine:
Blocks/binds H2-histamine receptors «in cells of stomach lining»
OR
prevents histamine molecules binding to H2-histamine receptors «and triggering acid secretion»
Omeprazole:
inhibits enzyme/gastric proton pump which secretes H+ ions «into gastric juice»
Accept “H2 receptor antagonist” for M1.
[2 marks]
[Na2CO3] = «\(\frac{{0.500{\text{ g}}}}{{105.99{\text{ g}}\,{\text{mo}}{{\text{l}}^{ – 1}} \times 0.075{\text{ d}}{{\text{m}}^3}}}\)=» 0.0629 «mol\(\,\)dm−3»
«pH = pKa + log\(\frac{{[{\text{conj base]}}}}{{[{\text{conj acid}}]}}\)»
«pH = 10.35 – 0.201 =» 10.15
Alternative method involving Ka may be used to deduce pH in M2.
Award [2] for correct final answer.
-2 marks]
Question
The structures of oseltamivir (Tamiflu) and zanamivir (Relenza) are given in section 37 of the data booklet.
Compare and contrast the structures of oseltamivir and zanamivir, stating the names of functional groups.
Deduce the wavenumber of one absorbance seen in the IR spectrum of only one of the compounds, using section 26 of the data booklet.
Suggest one ethical consideration faced by medical researchers when developing medications.
▶️Answer/Explanation
Markscheme
One similarity:
both contain amido «group»
One difference:
oseltamivir contains ester «group» AND zanamivir does not
OR
oseltamivir contains amino «group» AND zanamivir does not «but contains a guanidino group»
OR
zanamivir contains carboxyl «group» AND oseltamivir does not
OR
zanamivir contains «several» hydroxyl «groups» AND oseltamivir does not
OR
oseltamivir contains ester «group» AND zanamivir contains carboxyl «group»
OR
oseltamivir contains ester «group» AND zanamivir contains «several» hydroxyl «groups»
Accept “both contain ether «group»” OR “both contain alkene/alkenyl «group»” OR “both contain carbonyl «group»” OR “both contain amino/amine «group»”. Latter cannot be given in combination with second difference alternative with respect to amino group.
Accept “amide/carboxamide/carbamoyl” for “amido”.
Accept “amine” for “amino”.
Accept “carboxylic acid” for “carboxyl”.
Accept “hydroxy/alcohol” for “hydroxyl”, but not “hydroxide”.
[2 marks]
1050–1410
OR
1620–1680
OR
1700–1750
OR
2500–3000
OR
3200–3600
OR
2850–3090
OR
3300–3500 «cm–1»
[1 mark]
«negative» side-effects of medication on patient/volunteers
OR
effects on environment «from all materials used and produced»
OR
potential for abuse
OR
drugs may be developed that are contrary to some religious doctrines
OR
animal testing
OR
risk to benefit ratio
OR
appropriate consent of patient volunteers
[1 mark]
Question
Excess acid in the stomach can cause discomfort and more serious health issues.
Explain how ranitidine (Zantac) reduces stomach acid production.
The pH is maintained in different fluids in the body by the use of buffers.
Calculate the pH of a buffer solution of 0.0200 mol dm–3 carbonic acid, H2CO3, and 0.400 mol dm–3 sodium hydrogen carbonate, NaHCO3. The pKa of carbonic acid is 6.35.
▶️Answer/Explanation
Markscheme
blocks/binds to H2/histamine receptors «in cells of stomach lining»
OR
prevents histamine binding to H2/histamine receptors «and triggering acid secretion»
prevents parietal cells from releasing/producing acid
Accept “H2-receptor antagonist/H2RA” OR “blocks/inhibits action of histamine” for M1.
ALTERNATIVE 1
pH = «pKa + log \(\frac{{\left[ {{{\text{A}}^ – }} \right]}}{{\left[ {{\text{HA}}} \right]}}\) =» 6.35 + log (\(\frac{{0.400}}{{0.0200}}\))
«pH =» 7.65
ALTERNATIVE 2
Ka = 4.5 x 10–7
«Ka = 0.400 x \(\frac{{\left[ {{{\text{H}}^ + }} \right]}}{{0.0200}}\), [H+] =» 2.3 x 10–8 «mol dm–3»
«pH =» 7.64
Award [2] for correct final answer.
Do not accept “pH = 8”.
Question
Excess stomach acid can be counteracted by a range of medications.
An antacid tablet contains 680 mg of calcium carbonate, CaCO3, and 80 mg of magnesium carbonate, MgCO3.
State the equation for the reaction of magnesium carbonate with hydrochloric acid.
Determine the amount, in mol, of hydrochloric acid neutralized by one antacid tablet.
Explain how omeprazole (Prilosec) reduces stomach acidity.
▶️Answer/Explanation
Markscheme
MgCO3(s) + 2HCl(aq) → CO2(g) + H2O(l) + MgCl2(aq)
Do not accept “H2CO3”.
[1 mark]
n(HCl) = 2 n(CaCO3) + 2 n(MgCO3)
OR
n(HCl) = \(\frac{{2 \times 0.680\;\ll {\text{g}}\gg }}{{100.09\;\ll {\text{g mo}}{{\text{l}}^{ – 1}}\gg }} + \frac{{2 \times 0.080\;\ll {\text{g}}\gg }}{{84.32\;\ll {\text{g mo}}{{\text{l}}^{ – 1}}\gg }}\)
«n(HCl) = 0.0136 mol + 0.0019 mol =» 0.016 «mol»
Award [2] for correct final answer.
Award [1 max] for correctly calculating amount of acid neutralized by just CaCO3 (0.014 «mol») or MgCO3 (0.002 «mol»).
[2 marks]
inhibits the secretion of stomach acid/H+
«active metabolites» bind «irreversibly» to «receptors of the» proton pump
Accept “PPI/proton pump inhibitor”.
Do not award mark for “binds to H2/histamine receptors”. (Ranitidine mode of action.)
Accept “H+/K+ ATPase” for “proton pump”.
[2 marks]
Question
Excess acid in the stomach is often treated with calcium carbonate.
Formulate a chemical equation for the neutralization of stomach acid with calcium carbonate.
Calculate the amount, in mol, of stomach acid neutralized by an antacid tablet containing 0.750 g calcium carbonate.
Explain how omeprazole (Prilosec) regulates pH in the stomach.
▶️Answer/Explanation
Markscheme
2HCl(aq) + CaCO3(s) → H2O(l) + CO2(g) + CaCl2(aq)
Accept ionic equation:
2H+(aq) + CO32–(aq) → CO2(g) + H2O(l)
[1 mark]
«\(\frac{{0.750 \times 2}}{{100.09}}\) =» 0.0150 «mol HCl»
[1 mark]
inhibits the secretion of stomach acid/H+
«active metabolites» bind «irreversibly» to «receptors of the» proton pump
Do not accept “hydrogen/H/H2” for “H+”.
Accept “PPI/proton pump inhibitor” for M2.
Accept “H+/K+ ATPase” for “proton pump”.
[2 marks]

