Question
What is the percentage error if the enthalpy of combustion of a substance is determined experimentally to be -2100kJ \(mol^{-1}\) , but the literature value is -3500kJ \(mol^{-1}\)?
A. 80%
B. 60%
C. 40%
D. 20%
▶️Answer/Explanation
Markscheme: C
The percentage error is calculated using the formula:
\[ \text{Percentage Error} = \left| \frac{\text{Experimental Value} – \text{Literature Value}}{\text{Literature Value}} \right| \times 100\]
Given:
– Experimental Value = -2100 kJ/mol
– Literature Value = -3500 kJ/mol
\[ \text{Percentage Error} = \left| \frac{-2100 – (-3500)}{-3500} \right| \times 100\]
\[ \text{Percentage Error} = \left| \frac{1400}{3500} \right| \times 100\]
\[ \text{Percentage Error} = \frac{2}{5} \times 100\]
\[ \text{Percentage Error} = 40\%\]
Question
What is represented by the dotted line on the enthalpy profile?

A. Reaction carried out at a lower temperature
B. Reaction is reversible
C. A catalyst is used
D. Collision frequency has increased
Answer/Explanation
Markscheme: C
The dotted line on an enthalpy profile diagram typically represents the activation energy in a reaction. The activation energy is the minimum amount of energy required for a reaction to occur.
A catalyst is a substance that increases the rate of a chemical reaction by providing an alternative reaction pathway with a lower activation energy. The use of a catalyst does not affect the overall enthalpy change of the reaction, but it provides an alternative route for the reaction to occur more readily, often by providing a lower activation energy pathway. The dotted line represents this lower activation energy when a catalyst is present.
Question
What is the enthalpy of combustion of propan-1-ol, in kJ \(mol^{-1}\), according to the following calorimetry data?

A. \(\frac{-0.015 \times 4.2 \times 24}{0.015}\)
B. \(\frac{-75 \times 4.2 \times 24}{0.015}\)
C. \(\frac{-0.015 \times 4.2 \times 24}{75}\)
D. \(\frac{-75 \times 4.2 \times 24}{0.015 \times 1000}\)
▶️Answer/Explanation
Markscheme: D
The enthalpy change (\(\Delta H\)) for the combustion of propan-1-ol can be calculated using the formula:
\[
\Delta H = \frac{q}{n}
\]
where
\(q\) is the heat absorbed by the water,
\(n\) is the amount of substance (in moles).
The heat absorbed by the water (\(q\)) can be calculated using the formula:
\[
q = m \times c \times \Delta T
\]
where
\(m\) is the mass of water (in grams),
\(c\) is the specific heat capacity of water,
\(\Delta T\) is the temperature change.
Given data:
\[
\begin{align*}
m &= 75 \, \text{g} \\
c &= 4.2 \, \text{J g}^{-1} \, \text{K}^{-1} \\
\Delta T &= 24 \, ^{\circ} \text{C} = 24 \, \text{K} \\
n &= 0.015 \, \text{mol}
\end{align*}
\]
Now, substitute these values into the formulas:
\[
\begin{align*}
q &= 75 \times 4.2 \times 24 \\
\Delta H &= \frac{q}{n}
\end{align*}
\]
Now, substitute the values into the answer choices to find the correct one:
\[
\text{D. } \frac{-75 \times 4.2 \times 24}{0.015 \times 1000}
\]
This choice represents the correct calculation for the enthalpy of combustion of propan-1-ol. Therefore, the correct answer is D.
Question
Which processes have a negative enthalpy change?
I. \(2{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + 3{{\text{O}}_2}({\text{g)}} \to {\text{2C}}{{\text{O}}_2}({\text{g)}} + 4{{\text{H}}_2}{\text{O(l)}}\)
II. \({\text{HCl(aq)}} + {\text{NaOH(aq)}} \to {\text{NaCl(aq)}} + {{\text{H}}_2}{\text{O(l)}}\)
III. \({{\text{H}}_2}{\text{O(g)}} \to {{\text{H}}_2}{\text{O(l)}}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
D
Examiners report
Question
Which combination is correct for the exothermic reaction that occurs between zinc and copper sulfate solution.
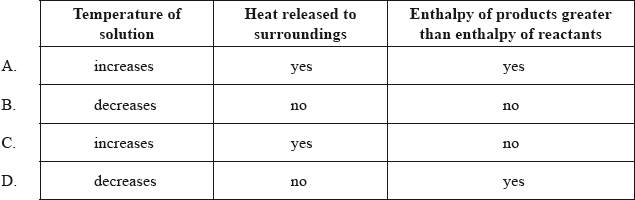
Answer/Explanation
Markscheme
C