Question
The periodic table provides information about electron configuration, and physical and chemical properties of elements.
(a) Bismuth has atomic number 83. Deduce two pieces of information about the electron configuration of bismuth from its position on the periodic table.
(b) Outline why aluminium is malleable.
(c) An 11.98g block of pure aluminium was heated. Calculate the heat energy absorbed, in J, to increase its temperature from 18.0°C to 40.0°C. The specific heat capacity of aluminium is 0.902J \(g^{−1} K^{−1}\)
(d) Argon has three naturally occurring isotopes, \(^{36}Ar\), \(^{38}Ar\) and \(^{40}Ar\).
(i) Identify the technique used to determine the relative proportions of the isotopes of argon.
The isotopic composition of a sample of argon is 0.34% of \(^{36}Ar\), 0.06% of \(^{38}Ar\) and 99.6% of \(^{40}Ar\).
(ii) Calculate the relative atomic mass of this sample, giving your answer to two
decimal places.
(e) State the full electron configuration of the cobalt(II) ion, \(Co^{2+}\)
Answer/Explanation
Answer:
(a) Any two of:
«group 15 so Bi has» 5 valence electrons
«period 6 so Bi has» 6 «occupied» electron shells/energy levels
«in p-block so» p orbitals are highest occupied
occupied d/f orbitals
has unpaired electrons
has incomplete shell(s)/subshell(s)
(b) «layers of» cations slide over each other without disrupting bonding
OR
attraction between metal ions and delocalized electrons/metallic bonding is not
disrupted by changing position of metal ions
OR
metallic bonds are non-directional
OR
changing the shape does not disrupt the bonding
(c) «heat energy = 11.98 g x 0.902 J \(g^{−1} K^{−1}\) x 22.0 K =» 238 «J»
(d) (i) mass spectrometry
OR
mass spectroscopy
OR
mass spectrum
OR
MS
(ii) (0.0034 x 36) + (0.0006 x 38) + (0.996 x 40)
39.99
(e) \(1s^2\) \(2s^2\) \(2p^6\) \(3s^2\) \(3p^6\) \(3d^7\)
Question
Methanol is made in large quantities as it is used in the production of polymers and in fuels. The enthalpy of combustion of methanol can be determined theoretically or experimentally.
\[{\text{C}}{{\text{H}}_3}{\text{OH(l)}} + {\text{1}}\frac{1}{2}{{\text{O}}_2}{\text{(g)}} \to {\text{C}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(g)}}\]
The enthalpy of combustion of methanol can also be determined experimentally in a school laboratory. A burner containing methanol was weighed and used to heat water in a test tube as illustrated below.
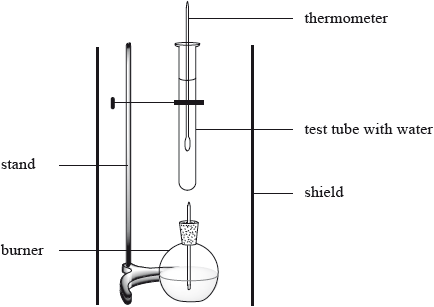
The following data were collected.

The Data Booklet value for the enthalpy of combustion of methanol is \( – {\text{726 kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\). Suggest why this value differs from the values calculated in parts (a) and (b).
Using the information from Table 10 of the Data Booklet, determine the theoretical enthalpy of combustion of methanol.
Calculate the amount, in mol, of methanol burned.
Calculate the heat absorbed, in kJ, by the water.
Determine the enthalpy change, in \({\text{kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}\), for the combustion of 1 mole of methanol.
Part (a)
Part (b)
Answer/Explanation
Markscheme
amount of energy required to break bonds of reactants
\(3 \times 413 + 358 + 464 + 1.5 \times 498{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}/2808{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
amount of energy released during bond formation of products
\(4 \times 464 + 2 \times 746{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}/3348{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
\(\Delta H = – 540{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
Award [3] for correct final answer.
Award [2] for (+)540.
If old Data Booklet is used accept answer: –535 (kJ\(\,\)mol–1) or award [2] for (+)535.
\(m{\text{(methanol)}} = (80.557 – 80.034) = 0.523{\text{ (g)}}\);
\(n{\text{(methanol)}} = \left( {\frac{{0.523{\text{ g}}}}{{32.05{\text{ g}}\,{\text{mo}}{{\text{l}}^{ – 1}}}}} \right) = 0.0163{\text{ (mol)}}\);
Award [2] for correct final answer.
\(\Delta T = (26.4 – 21.5) = 4.9{\text{ (K)}}\);
\(q = (mc\Delta T = ){\text{ }}20.000 \times 4.18 \times 4.9{\text{ (J)}}/20.000 \times 4.18 \times 4.9 \times {10^{ – 3}}{\text{ (kJ)}}\);
0.41 (kJ);
Award [3] for correct final answer.
\(\Delta H_{\text{c}}^\Theta = – \frac{{0.41{\text{ (kJ)}}}}{{0.0163{\text{ (mol)}}}}/ – 25153{\text{ (J}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
\( = – 25{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ – 1}}{\text{)}}\);
Award [2] for correct final answer.
Award [1] for (+)25 (kJ\(\,\)mol–1).
bond enthalpies are average values/differ (slightly) from one compound to another (depending on the neighbouring atoms) / methanol is liquid not gas in the reaction;
not all heat produced transferred to water / heat lost to surroundings/environment / OWTTE / incomplete combustion (of methanol) / water forms as \({\text{ }}{{\text{H}}_2}{\text{O(l)}}\) instead of \({{\text{H}}_2}{\text{O(g)}}\);
Do not allow just “heat lost”.
Examiners report
Many errors were seen in part (a). Candidates used the wrong values from the Data Booklet, wrong coefficients were used and not all the correct bonds were selected. Some candidates also reversed the final calculation to get an endothermic enthalpy rather than an exothermic enthalpy or made careless arithmetic errors.
Candidates were proficient at correctly calculating the number of mole methanol burnt.
Candidates did not use the expression \(q = mc\Delta T\) well.
Again numerous errors were seen here with candidates using the mass of methanol rather than water, adding 273 to the temperature change calculated and not converting J to kJ. Some candidates did not recognise that the combustion of methanol is exothermic and hence did not include the negative sign for the enthalpy change.
Part (c) was generally well done, however candidates often just stated that ‘heat was lost’ in part (ii). A more detail response was expected, e.g. heat was lost to surroundings.
Part (c) was generally well done, however candidates often just stated that ‘heat was lost’ in part (ii). A more detail response was expected, e.g. heat was lost to surroundings.