Question
Which diagram shows the enthalpy change for dissolving solid, X, in water, if the temperature of the solution decreases?
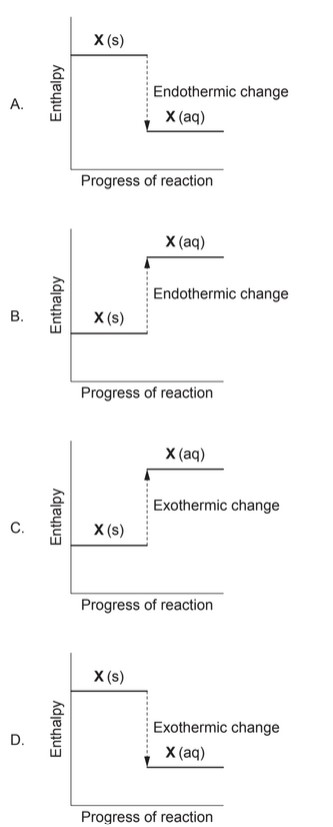
Answer/Explanation
Markscheme: B
Question
Which statement concerning bond breaking is correct?
A. Requires energy and is endothermic.
B. Requires energy and is exothermic.
C. Releases energy and is endothermic.
D. Releases energy and is exothermic.
▶️Answer/Explanation
Markscheme: A
Breaking a chemical bond requires an input of energy to overcome the attractive forces between atoms. Therefore, the correct statement concerning bond breaking is:
A. Requires energy and is endothermic.
Breaking bonds is an endothermic process because energy is absorbed to break the existing bonds. It is the opposite of bond formation, which is exothermic, where energy is released.
Question
B. \(\frac{1}{4}\) SiH4 (g) → \(\frac{1}{4}\) Si(g) + H(g)
C. SiH4(g) → SiH3(g) + \(\frac{1}{2}\) H2(g)
D. SiH4 (g) → Si(g) + 4H(g)
Answer/Explanation
Markscheme
B
Examiners report
Question
What can be deduced from the facts that ozone absorbs UV radiation in the region of 340 nm and molecular oxygen in the region of 242 nm?
A. The bond between atoms in molecular oxygen is a double bond.
B. The bonds in ozone are delocalized.
C. The bonds between atoms in ozone are stronger than those in molecular oxygen.
D. The bonds between atoms in molecular oxygen need more energy to break.
Answer/Explanation
Markscheme
D
Examiners report
Question
Which describes the reaction shown in the potential energy profile?
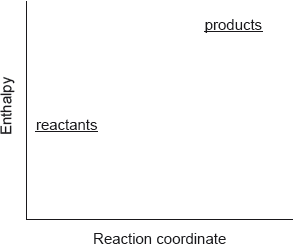
A. The reaction is endothermic and the products have greater enthalpy than the reactants.
B. The reaction is endothermic and the reactants have greater enthalpy than the products.
C. The reaction is exothermic and the products have greater enthalpy than the reactants.
D. The reaction is exothermic and the reactants have greater enthalpy than the products.
Answer/Explanation
Markscheme
A