Question
The following graph shows the concentration of \(C_4H_9Cl\) versus time.

What is the average rate of reaction over the first 800 seconds?
A. \(1 × 10^{-3}\)mol \(dm^{-3} s^{-1}\)
B. \(1 × 10^{-4}\)mol \(dm^{-3} s^{-1}\)
C. \(2 × 10^{-3}\)mol \(dm^{-3} s^{-1}\)
D. \(2 × 10^{-4}\)mol \(dm^{-3} s^{-1}\)
▶️Answer/Explanation
Markscheme: B
\[
\begin{aligned}
&\text { rate }=-\frac{\text { [reactant at } \left.\mathrm{t}_2\right]-\left[\text { reactant at } \mathrm{t}_1\right]}{\mathrm{t}_2-\mathrm{t}_1}
\end{aligned}
\]
\[
\begin{aligned}&\text { rate }=-\frac{ { [ } \left.0.10\right]- { [ } \left.0.02\right]}{0-800}\Rightarrow 1\times 10^{-4}\end{aligned}
\]
Question
Consider the reaction between gaseous iodine and gaseous hydrogen.
\[\begin{array}{*{20}{l}} {{{\text{I}}_2}{\text{(g)}} + {{\text{H}}_2}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}}&{\Delta {H^\Theta } = – 9{\text{ kJ}}} \end{array}\]
Why do some collisions between iodine and hydrogen not result in the formation of the product?
A. The \({{\text{I}}_{\text{2}}}\) and \({{\text{H}}_{\text{2}}}\) molecules do not have sufficient energy.
B. The system is in equilibrium.
C. The temperature of the system is too high.
D. The activation energy for this reaction is very low.
Answer/Explanation
Markscheme
A
Examiners report
Question
Which statement about the kinetic theory is not correct?
A. The particles in ice vibrate about fixed points.
B. The particles in steam have more energy than the particles in ice.
C. All the particles in water have the same amount of energy at 298 K.
D. Evaporation of water occurs at all temperatures between 273 K and 373 K when the atmospheric pressure is 101 kPa.
Answer/Explanation
Markscheme
C
Examiners report
There were two G2 comments on this question both of which stated that this was a difficult question at SL. The question certainly was challenging and was found to be the third hardest question on the paper. However, 49% of candidates did manage to get the correct answer, C.
Question
The following enthalpy level diagram shows the effect of the addition of a catalyst on a chemical reaction. What do m, n and o represent?
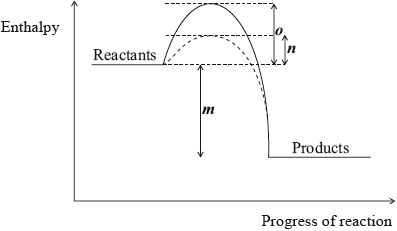
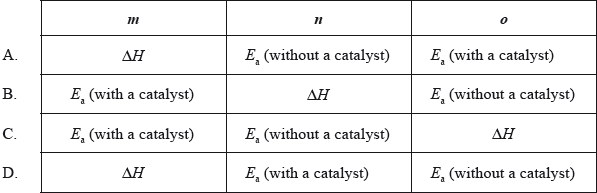
Answer/Explanation
Markscheme
D