Question
Which value of the reaction quotient, Q, shows the lowest relative amount of products at a particular point in time?
A. \(4.9 × 10^{-3}\)
B. \(8.2 × 10^{-3}\)
C. \(4.9 × 10^2\)
D. \(8.2 × 10^2\)
▶️Answer/Explanation
Markscheme: A
The reaction quotient (\(Q\)) is the ratio of the concentrations of products to reactants in a chemical reaction at any given point in time. To determine which value of \(Q\) shows the lowest relative amount of products, we need to consider the reaction’s equilibrium constant (\(K\)).
If \(Q < K\), the reaction will shift to the right to reach equilibrium, favoring the formation of more products. If \(Q > K\), the reaction will shift to the left, favoring the formation of more reactants.
So, the lowest relative amount of products will be associated with the smallest value of \(Q\) among the options provided. Therefore, the correct choice is:
A. \(4.9 × 10^{-3}\)
Question
Which condition will cause the given equilibrium to shift to the right?
\(Ag^+(aq) + Cl^-(aq) \rightleftharpoons AgCl(s)\)
A. One half of solid AgCl is removed.
B. Water is added.
C. Solid NaCl is added.
D. The system is subjected to increased pressure.
▶️Answer/Explanation
Markscheme: C
To predict the effect on the equilibrium position, we can use Le Chatelier’s principle, which states that if a system at equilibrium is subjected to a change, the system will adjust itself to counteract that change.
In the given equilibrium:
\[ \text{Ag}^+(aq) + \text{Cl}^-(aq) \rightleftharpoons \text{AgCl}(s) \]
Let’s consider each option:
A. One half of solid AgCl is removed: This is equivalent to removing a product. According to Le Chatelier’s principle, the system will shift to the right to counteract the loss of product. Therefore, the equilibrium will shift to the right.
B. Water is added: Adding water doesn’t affect the concentrations of the ions or the solid. It’s essentially a change in volume, but it won’t shift the equilibrium to the right.
C. Solid NaCl is added: Since NaCl dissociates into Na⁺ and Cl⁻ ions in the solution, adding solid NaCl introduces more Cl⁻ ions. According to Le Chatelier’s principle, the system will shift to the right to counteract the increase in Cl⁻ ions. Therefore, the equilibrium will shift to the right.
D. The system is subjected to increased pressure: This system involves only aqueous ions and a solid, so pressure changes won’t have a significant effect on the equilibrium position. It’s not a factor that would cause a shift to the right.
Therefore, the correct answer is C. Solid NaCl is added.
Question
Which is always correct for a reaction at equilibrium?
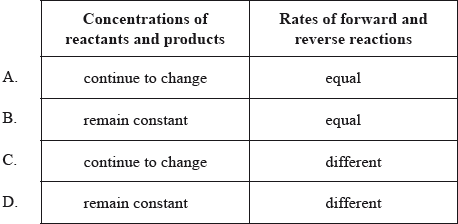
Answer/Explanation
Markscheme
B
Examiners report
Question
What is the equilibrium constant expression, \({K_{\text{c}}}\), for the formation of hydrogen iodide from its elements?
\[{{\text{H}}_{\text{2}}}{\text{(g)}} + {{\text{I}}_{\text{2}}}{\text{(g)}} \rightleftharpoons {\text{2HI(g)}}\]
A. \({K_{\text{c}}} = \frac{{{{{\text{[HI]}}}^2}}}{{{\text{[}}{{\text{H}}_2}{\text{]}} \times {\text{[}}{{\text{I}}_2}{\text{]}}}}\)
B. \({K_{\text{c}}} = \frac{{{\text{[2HI]}}}}{{{\text{[}}{{\text{H}}_2}{\text{]}} + {\text{[}}{{\text{I}}_2}{\text{]}}}}\)
C. \({K_{\text{c}}} = \frac{{2{{{\text{[HI]}}}^2}}}{{{\text{[}}{{\text{H}}_2}{\text{]}} + {\text{[}}{{\text{I}}_2}{\text{]}}}}\)
D. \({K_{\text{c}}} = \frac{{{\text{[2HI]}}}}{{{\text{[}}{{\text{H}}_2}{\text{]}} \times {\text{[}}{{\text{I}}_2}{\text{]}}}}\)
Answer/Explanation
Markscheme
A
Examiners report
\({K_{\text{c}}}\) expressions often leave much to be desired so it was encouraging to see that this was the easiest question on the paper with 93% of answers correct.
Question
Consider the equilibrium between N2O4(g) and NO2(g).
N2O4(g) \( \rightleftharpoons \) 2NO2(g) ΔH = +58 kJ\(\,\)mol−1
Which changes shift the position of equilibrium to the right?
\(\begin{array}{*{20}{l}} {{\text{I.}}}&{{\text{Increasing the temperature}}} \\ {{\text{II.}}}&{{\text{Decreasing the pressure}}} \\ {{\text{III.}}}&{{\text{Adding a catalyst}}} \end{array}\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Answer/Explanation
Markscheme
A