Question
Which combination describes a strong Brønsted–Lowry acid?

▶️Answer/Explanation
Markscheme: B
A strong Brønsted–Lowry acid is a substance that easily donates protons (H⁺ ions). Its conjugate base is weak because it doesn’t readily accept protons. So, for a strong Brønsted–Lowry acid:
1. Proton Donor: The strong acid easily donates protons. Therefore, the proton donor is the strong acid.
2. Conjugate Base: The conjugate base is formed when the strong acid donates a proton. Since the acid is strong, its conjugate base is weak. Strong acids typically have weak conjugate bases.
In general terms, a strong Brønsted–Lowry acid can be represented as follows:
Proton Donor: Strong Acid
Conjugate Base: Weak Base
Question
Which reaction represents the neutralization of a Brønsted–Lowry acid and base?
A. \(2HCl(aq) + Zn(s) → ZnCl_2 (aq) + H_2 (g)\)
B. \(2HCl(aq) + ZnO(s) → ZnCl_2 (aq) + H_2O(l)\)
C. \(4NH_3 (g) + 5O_2 (g) → 4NO(g) + 6H_2O(l)\)
D. \(C_2H_4 (g) + H_2 (g) → C_2H_6 (g)\)
▶️Answer/Explanation
Markscheme: B
A neutralization reaction involves the combination of an acid and a base to form water and a salt. In the context of Brønsted–Lowry acid-base theory, the acid donates a proton (H⁺) to the base.
Let’s analyze the options:
A. \(2HCl(aq) + Zn(s) → ZnCl_2 (aq) + H_2 (g)\): This reaction involves hydrochloric acid reacting with zinc to produce zinc chloride and hydrogen gas. It is not a neutralization reaction; it is a single-replacement reaction.
B. \(2HCl(aq) + ZnO(s) → ZnCl_2 (aq) + H_2O(l)\): This reaction involves hydrochloric acid reacting with zinc oxide to produce zinc chloride and water. This is a neutralization reaction, as water is formed.
C. \(4NH_3 (g) + 5O_2 (g) → 4NO(g) + 6H_2O(l)\): This reaction involves the combustion of ammonia with oxygen to produce nitrogen monoxide and water. It is not a neutralization reaction.
D. \(C_2H_4 (g) + H_2 (g) → C_2H_6 (g)\): This reaction involves the addition of hydrogen to ethene to form ethane. It is not a neutralization reaction.
Therefore, the correct answer is:
B. \(2HCl(aq) + ZnO(s) → ZnCl_2 (aq) + H_2O(l)\)
Question
Which row correctly describes \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ – 3}}{\text{ NaOH(aq)}}\)?
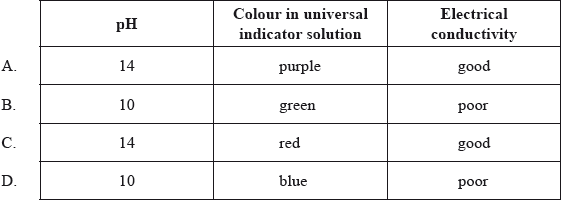
Answer/Explanation
Markscheme
A
Examiners report
There was concern expressed that we expect candidates to know the colours of universal indicator by rote learning. Far from it, we would expect candidates to have absorbed this information during regular lab classes and demonstrations.
Question
Which species behave as Brønsted–Lowry bases in the following reaction?
H2SO4 + HNO3 H2NO3+ + HSO4–
A. HNO3 and HSO4–
B. HNO3 and H2NO3+
C. H2SO4 and HSO4–
D. H2NO3+ and HSO4–
Answer/Explanation
Markscheme
A
Examiners report
Question
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A Cu2+
B NH4 +
C Cu
D CH3COOH
Answer/Explanation
Ans: A